Control of Clostridium difficile Infection by Defined Microbial Communities
- PMID: 28936948
- PMCID: PMC5736378
- DOI: 10.1128/microbiolspec.BAD-0009-2016
Control of Clostridium difficile Infection by Defined Microbial Communities
Abstract
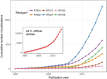
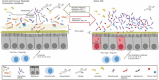
Each year in the United States, billions of dollars are spent combating almost half a million Clostridium difficile infections (CDIs) and trying to reduce the ∼29,000 patient deaths in which C. difficile has an attributed role. In Europe, disease prevalence varies by country and level of surveillance, though yearly costs are estimated at €3 billion. One factor contributing to the significant health care burden of C. difficile is the relatively high frequency of recurrent CDIs. Recurrent CDI, i.e., a second episode of symptomatic CDI occurring within 8 weeks of successful initial CDI treatment, occurs in ∼25% of patients, with 35 to 65% of these patients experiencing multiple episodes of recurrent disease. Using microbial communities to treat recurrent CDI, either as whole fecal transplants or as defined consortia of bacterial isolates, has shown great success (in the case of fecal transplants) or potential promise (in the case of defined consortia of isolates). This review will briefly summarize the epidemiology and physiology of C. difficile infection, describe our current understanding of how fecal microbiota transplants treat recurrent CDI, and outline potential ways that knowledge can be used to rationally design and test alternative microbe-based therapeutics.
Figures
References
-
- Lessa FC, Mu Y, Bamberg WM, Beldavs ZG, Dumyati GK, Dunn JR, Farley MM, Holzbauer SM, Meek JI, Phipps EC, Wilson LE, Winston LG, Cohen JA, Limbago BM, Fridkin SK, Gerding DN, McDonald LC. 2015. Burden of Clostridium difficile infection in the United States. N Engl J Med 372:2369–2370 10.1056/NEJMoa1408913. 10.1056/NEJMoa1408913 - DOI - DOI - PMC - PubMed
-
- Shah DN, Aitken SL, Barragan LF, Bozorgui S, Goddu S, Navarro ME, Xie Y, DuPont HL, Garey KW. 2016. Economic burden of primary compared with recurrent Clostridium difficile infection in hospitalized patients: a prospective cohort study. J Hosp Infect 93:286–289 10.1016/j.jhin.2016.04.004. [PubMed]10.1016/j.jhin.2016.04.004 - DOI - DOI - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

