Priming of the Cells: Hypoxic Preconditioning for Stem Cell Therapy
- PMID: 28937044
- PMCID: PMC5634089
- DOI: 10.4103/0366-6999.215324
Priming of the Cells: Hypoxic Preconditioning for Stem Cell Therapy
Abstract
Objective: Stem cell-based therapies are promising in regenerative medicine for protecting and repairing damaged brain tissues after injury or in the context of chronic diseases. Hypoxia can induce physiological and pathological responses. A hypoxic insult might act as a double-edged sword, it induces cell death and brain damage, but on the other hand, sublethal hypoxia can trigger an adaptation response called hypoxic preconditioning or hypoxic tolerance that is of immense importance for the survival of cells and tissues.
Data sources: This review was based on articles published in PubMed databases up to August 16, 2017, with the following keywords: "stem cells," "hypoxic preconditioning," "ischemic preconditioning," and "cell transplantation."
Study selection: Original articles and critical reviews on the topics were selected.
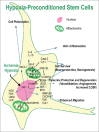
Results: Hypoxic preconditioning has been investigated as a primary endogenous protective mechanism and possible treatment against ischemic injuries. Many cellular and molecular mechanisms underlying the protective effects of hypoxic preconditioning have been identified.
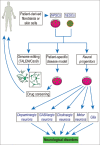
Conclusions: In cell transplantation therapy, hypoxic pretreatment of stem cells and neural progenitors markedly increases the survival and regenerative capabilities of these cells in the host environment, leading to enhanced therapeutic effects in various disease models. Regenerative treatments can mobilize endogenous stem cells for neurogenesis and angiogenesis in the adult brain. Furthermore, transplantation of stem cells/neural progenitors achieves therapeutic benefits via cell replacement and/or increased trophic support. Combinatorial approaches of cell-based therapy with additional strategies such as neuroprotective protocols, anti-inflammatory treatment, and rehabilitation therapy can significantly improve therapeutic benefits. In this review, we will discuss the recent progress regarding cell types and applications in regenerative medicine as well as future applications.
Conflict of interest statement
There are no conflicts of interest.
Figures
Similar articles
-
Hypoxic preconditioning enhances neural stem cell transplantation therapy after intracerebral hemorrhage in mice.J Cereb Blood Flow Metab. 2016 Dec;36(12):2134-2145. doi: 10.1177/0271678X15613798. Epub 2015 Oct 27. J Cereb Blood Flow Metab. 2016. PMID: 26661220 Free PMC article.
-
Preconditioning strategy in stem cell transplantation therapy.Transl Stroke Res. 2013 Feb;4(1):76-88. doi: 10.1007/s12975-012-0251-0. Transl Stroke Res. 2013. PMID: 23914259 Free PMC article. Review.
-
In vitro hypoxic preconditioning of embryonic stem cells as a strategy of promoting cell survival and functional benefits after transplantation into the ischemic rat brain.Exp Neurol. 2008 Apr;210(2):656-70. doi: 10.1016/j.expneurol.2007.12.020. Epub 2008 Jan 5. Exp Neurol. 2008. PMID: 18279854
-
Priming adult stem cells by hypoxic pretreatments for applications in regenerative medicine.J Biomed Sci. 2013 Aug 29;20(1):63. doi: 10.1186/1423-0127-20-63. J Biomed Sci. 2013. PMID: 23985033 Free PMC article. Review.
-
Stem cell transplantation therapy for multifaceted therapeutic benefits after stroke.Prog Neurobiol. 2017 Oct;157:49-78. doi: 10.1016/j.pneurobio.2017.03.003. Epub 2017 Mar 18. Prog Neurobiol. 2017. PMID: 28322920 Free PMC article. Review.
Cited by
-
Hypoxia-Preconditioned Placenta-Derived Mesenchymal Stem Cells Rescue Optic Nerve Axons Via Differential Roles of Vascular Endothelial Growth Factor in an Optic Nerve Compression Animal Model.Mol Neurobiol. 2020 Aug;57(8):3362-3375. doi: 10.1007/s12035-020-01965-8. Epub 2020 Jun 10. Mol Neurobiol. 2020. PMID: 32524519
-
Nano hydrogel-based oxygen-releasing stem cell transplantation system for treating diabetic foot.J Nanobiotechnology. 2023 Jun 27;21(1):202. doi: 10.1186/s12951-023-01925-z. J Nanobiotechnology. 2023. PMID: 37370102 Free PMC article.
-
Hypoxic pre-conditioned adipose-derived stem/progenitor cells embedded in fibrin conduits promote peripheral nerve regeneration in a sciatic nerve graft model.Neural Regen Res. 2023 Mar;18(3):652-656. doi: 10.4103/1673-5374.346464. Neural Regen Res. 2023. PMID: 36018190 Free PMC article.
-
In vivo priming of human mesenchymal stem cells with hepatocyte growth factor-engineered mesenchymal stem cells promotes therapeutic potential for cardiac repair.Sci Adv. 2020 Mar 25;6(13):eaay6994. doi: 10.1126/sciadv.aay6994. eCollection 2020 Mar. Sci Adv. 2020. PMID: 32284967 Free PMC article.
-
Glial Cell-Based Vascular Mechanisms and Transplantation Therapies in Brain Vessel and Neurodegenerative Diseases.Front Cell Neurosci. 2021 Mar 26;15:627682. doi: 10.3389/fncel.2021.627682. eCollection 2021. Front Cell Neurosci. 2021. PMID: 33841101 Free PMC article. Review.
References
-
- Wei L, Yu SP, Gottron F, Snider BJ, Zipfel GJ, Choi DW. Potassium channel blockers attenuate hypoxia-and ischemia-induced neuronal death in vitro and in vivo. Stroke. 2003;34:1281–6. doi: 10.1161/01.str.0000065828.18661.fe. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
