Remotely supervised transcranial direct current stimulation for the treatment of fatigue in multiple sclerosis: Results from a randomized, sham-controlled trial
- PMID: 28937310
- PMCID: PMC5975187
- DOI: 10.1177/1352458517732842
Remotely supervised transcranial direct current stimulation for the treatment of fatigue in multiple sclerosis: Results from a randomized, sham-controlled trial
Abstract
Background: Fatigue is a common and debilitating feature of multiple sclerosis (MS) that remains without reliably effective treatment. Transcranial direct current stimulation (tDCS) is a promising option for fatigue reduction. We developed a telerehabilitation protocol that delivers tDCS to participants at home using specially designed equipment and real-time supervision (remotely supervised transcranial direct current stimulation (RS-tDCS)).
Objective: To evaluate whether tDCS can reduce fatigue in individuals with MS.
Methods: Dorsolateral prefrontal cortex left anodal tDCS was administered using a RS-tDCS protocol, paired with 20 minutes of cognitive training. Here, two studies are considered. Study 1 delivered 10 open-label tDCS treatments (1.5 mA; n = 15) compared to a cognitive training only condition ( n = 20). Study 2 was a randomized trial of active (2.0 mA, n = 15) or sham ( n = 12) delivered for 20 sessions. Fatigue was assessed using the Patient-Reported Outcomes Measurement Information System (PROMIS)-Fatigue Short Form.
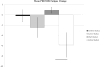
Results and conclusion: In Study 1, there was modest fatigue reduction in the active group (-2.5 ± 7.4 vs -0.2 ± 5.3, p = 0.30, Cohen's d = -0.35). However, in Study 2 there was statistically significant reduction for the active group (-5.6 ± 8.9 vs 0.9 ± 1.9, p = 0.02, Cohen's d = -0.71). tDCS is a potential treatment for MS-related fatigue.
Keywords: Fatigue; multiple sclerosis; tDCS; tES; telerehabilitation; transcranial direct current stimulation.
Conflict of interest statement
The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: CUNY has patents with M.B. as inventor. M.B is an advisor for and has equity in Soterix Medical. CUNY has patents with A.D. as inventor. A.D. is an employee and has equity in Soterix Medical.
Figures


References
-
- Poser CM, Brinar VV. The accuracy of prevalence rates of multiple sclerosis: A critical review. Neuroepidemiology. 2007;29(3–4):150–155. - PubMed
-
- Krupp L. Fatigue is intrinsic to multiple sclerosis (MS) and is the most commonly reported symptom of the disease. Mult Scler. 2006;12(4):367–368. - PubMed
-
- Lerdal A, Celius EG, Krupp L, et al. A prospective study of patterns of fatigue in multiple sclerosis. Eur J Neurol. 2007;14(12):1338–1343. - PubMed
-
- Kurtzke JF. Rating neurologic impairment in multiple sclerosis: An expanded disability status scale (EDSS) Neurology. 1983;33(11):1444–1452. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

