Aspalathin Reverts Doxorubicin-Induced Cardiotoxicity through Increased Autophagy and Decreased Expression of p53/mTOR/p62 Signaling
- PMID: 28937626
- PMCID: PMC6151817
- DOI: 10.3390/molecules22101589
Aspalathin Reverts Doxorubicin-Induced Cardiotoxicity through Increased Autophagy and Decreased Expression of p53/mTOR/p62 Signaling
Abstract
Doxorubicin (Dox) is an effective chemotherapeutic agent used in the treatment of various cancers. Its clinical use is often limited due to its potentially fatal cardiotoxic side effect. Increasing evidence indicates that tumour protein p53 (p53), adenosine monophosphate-activated protein kinase (AMPK), nucleoporin p62 (p62), and the mammalian target of rapamycin (mTOR) are critical mediators of Dox-induced apoptosis, and subsequent dysregulation of autophagy. Aspalathin, a polyphenolic dihydrochalcone C-glucoside has been shown to activate AMPK while decreasing the expression of p53. However, the role that aspalathin could play in the inhibition of Dox-induced cardiotoxicity through increased autophagy flux remained unexplored. H9c2 cardiomyocytes and Caov-3 ovarian cancer cells were cultured in Dulbecco's Modified Eagle's medium and treated with or without Dox for five days. Thereafter, cells exposed to 0.2 µM Dox were co-treated with either 20 µM Dexrazozane (Dexra) or 0.2 µM aspalathin (ASP) daily for 5 days. Results obtained showed that ASP mediates its cytoprotective effect in a p53-dependent manner, by increasing the Bcl-2/Bax ratio and decreasing apoptosis. The latter effect was diminished through ASP-induced activation of autophagy-related genes (Atgs) with an associated decrease in p62 through induction of AMPK and Fox01. Furthermore, we showed that ASP was able to potentiate this effect without decreasing the anti-cancer efficacy of Dox, as could be observed in Caov-3 ovarian cancer cells. Taken together, the data presented in this study provides a credible mechanism by which ASP co-treatment could protect the myocardium from Dox-induced cardiotoxicity.
Keywords: apoptosis; aspalathin; autophagy; cardiomyopathy; cardiotoxicity; doxorubicin; oxidative stress.
Conflict of interest statement
The authors declare no conflict of interest.
Figures





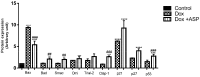
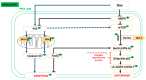
References
-
- Bjorkoy G., Lamark T., Pankiv S., Overvatn A., Brech A., Johansen T. Monitoring autophagic degradation of p62/sqstm1. Methods Enzymol. 2009;452:181–197. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

