Total synthesis of (-)-tubingensin B enabled by the strategic use of an aryne cyclization
- PMID: 28937679
- PMCID: PMC6994261
- DOI: 10.1038/nchem.2801
Total synthesis of (-)-tubingensin B enabled by the strategic use of an aryne cyclization
Abstract
Tubingensin B is an indole diterpenoid that bears a daunting chemical structure featuring a disubstituted carbazole unit, five stereogenic centres-three of which are quaternary-and a decorated [3.2.2]-bridged bicycle. We describe our synthetic design toward a concise and enantiospecific total synthesis of tubingensin B, which hinges on the strategic use of a transient aryne intermediate. Although initial studies led to unexpected reaction outcomes, we ultimately implemented a sequence of carbazolyne cyclization followed by Rh-catalysed fragmentation to install the seven-membered ring and vicinal quaternary stereocentres of the natural product. Coupled with a late-stage radical cyclization to construct the [3.2.2]-bridged bicycle, these efforts have enabled the total synthesis of tubingensin B. The design and evolution of our succinct total synthesis underscores the utility of long-avoided aryne intermediates for the introduction of structural motifs that have conventionally been viewed as challenging.
Conflict of interest statement
Competing financial interests
The authors declare no competing financial interests.
Figures

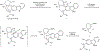




References
-
- TePaske MR, Gloer JB, Wicklow DT & Dowd PF The structure of tubingensin B: a cytotoxic carbazole alkaloid from the sclerotia of Aspergillus tubingensis. Tetrahedron Lett. 30, 5965–5968 (1989).
-
- Swenson DC, TePaske MR, Baenziger NC & Gloer JB Tubingensin B, a cytotoxic carbazole alkaloid from the sclerotia of Aspergillus tubingensis. Acta Cryst. C 53, 1447–1449 (1997).
-
- Bian M et al. Total synthesis of anominine and tubingensin A. J. Am. Chem. Soc 134, 8078–8081 (2012). - PubMed
-
- Goetz AE, Shah TK & Garg NK Pyridynes and indolynes as building blocks for functionalized heterocycles and natural products. Chem. Commun 51, 34–45 (2015). - PubMed
Publication types
MeSH terms
Substances
Associated data
- PubChem-Substance/336447057
- PubChem-Substance/336447077
- PubChem-Substance/336447067
- PubChem-Substance/336447074
- PubChem-Substance/336447075
- PubChem-Substance/336447076
- PubChem-Substance/336447058
- PubChem-Substance/336447078
- PubChem-Substance/336447059
- PubChem-Substance/336447060
- PubChem-Substance/336447061
- PubChem-Substance/336447062
- PubChem-Substance/336447063
- PubChem-Substance/336447064
- PubChem-Substance/336447065
- PubChem-Substance/336447066
- PubChem-Substance/336447068
- PubChem-Substance/336447069
- PubChem-Substance/336447070
- PubChem-Substance/336447071
- PubChem-Substance/336447072
- PubChem-Substance/336447073
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

