Interventions for treating genital Chlamydia trachomatis infection in pregnancy
- PMID: 28937705
- PMCID: PMC6483758
- DOI: 10.1002/14651858.CD010485.pub2
Interventions for treating genital Chlamydia trachomatis infection in pregnancy
Abstract
Background: Genital Chlamydia trachomatis (C.trachomatis) infection may lead to pregnancy complications such as miscarriage, preterm labour, low birthweight, preterm rupture of membranes, increased perinatal mortality, postpartum endometritis, chlamydial conjunctivitis and C.trachomatis pneumonia.This review supersedes a previous review on this topic.
Objectives: To establish the most efficacious and best-tolerated therapy for treatment of genital chlamydial infection in preventing maternal infection and adverse neonatal outcomes.
Search methods: We searched the Cochrane Pregnancy and Childbirth Group's Trials Register, ClinicalTrials.gov, the WHO International Clinical Trials Registry Platform (ICTRP) (26 June 2017) and reference lists of retrieved studies.
Selection criteria: Randomised controlled trials (RCTs) as well as studies published in abstract form assessing interventions for treating genital C.trachomatis infection in pregnancy. Cluster-RCTs were also eligible for inclusion but none were identified. Quasi-randomised trials and trials using cross-over design are not eligible for inclusion in this review.
Data collection and analysis: Two review authors independently assessed studies for inclusion, assessed trial quality and extracted the data using the agreed form. Data were checked for accuracy. Evidence was assessed using the GRADE approach.
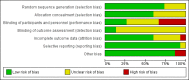
Main results: We included 15 trials (involving 1754 women) although our meta-analyses were based on fewer numbers of studies/women. All of the included studies were undertaken in North America from 1982 to 2001. Two studies were low risk of bias in all domains, all other studies had varying risk of bias. Four other studies were excluded and one study is ongoing.Eight comparisons were included in this review; three compared antibiotic (erythromycin, clindamycin, amoxicillin) versus placebo; five compared an antibiotic versus another antibiotic (erythromycin, clindamycin, amoxicillin, azithromycin). No study reported different antibiotic regimens. Microbiological cure (primary outcome) Antibiotics versus placebo: Erythromycin (average risk ratio (RR) 2.64, 95% confidence interval (CI) 1.60 to 4.38; two trials, 495 women; I2 = 68%; moderate-certainty evidence), and clindamycin (RR 4.08, 95% CI 2.35 to 7.08; one trial, 85 women;low-certainty evidence) were associated with improved microbiological cure compared to a placebo control. In one very small trial comparing amoxicillin and placebo, the results were unclear, but the evidence was graded very low (RR 2.00, 95% CI 0.59 to 6.79; 15 women). One antibiotic versus another antibiotic: Amoxicillin made little or no difference in microbiological cure in comparison to erythromycin (RR 0.97, 95% CI 0.93 to 1.01; four trials, 466 women; high-certainty evidence), probably no difference compared to clindamycin (RR 0.96, 95% CI 0.89 to 1.04; one trial, 101 women; moderate-quality evidence), and evidence is very low certainty when compared to azithromycin so the effect is not certain (RR 0.89, 95% CI 0.71 to 1.12; two trials, 144 women; very low-certainty evidence). Azithromycin versus erythromycin (average RR 1.11, 95% CI 1.00 to 1.23; six trials, 374 women; I2 = 53%; moderate-certainty evidence) probably have similar efficacy though results appear to favour azithromycin. Clindamycin versus erythromycin (RR 1.06, 95% CI 0.97 to 1.15; two trials, 173 women; low-certainty evidence) may have similar numbers of women with a microbiological cure between groups.Evidence was downgraded for design limitations, inconsistency, and imprecision in effect estimates. Side effects of the treatment (maternal) (secondary outcome) Antibiotics versus placebo: side effects including nausea, vomiting, and abdominal pain, were reported in two studies (495 women) but there was no clear evidence whether erythromycin was associated with more side effects than placebo and a high level of heterogeneity (I2 = 78%) was observed (average RR 2.93, 95% CI 0.36 to 23.76). There was no clear difference in the number of women experiencing side effects when clindamycin was compared to placebo in one small study (5/41 versus 1/44) (RR 6.35, 95% CI 0.38 to 107.45, 62 women). The side effects reported were mostly gastrointestinal and also included resolving skin rashes. One antibiotic versus another antibiotic: There was no clear difference in incidence of side effects (including nausea, vomiting, diarrhoea and abdominal pain) when amoxicillin was compared to azithromycin based on data from one small study (36 women) (RR 0.56, 95% CI 0.24 to 1.31).However, amoxicillin was associated with fewer side effects compared to erythromycin with data from four trials (513 women) (RR 0.31, 95% CI 0.21 to 0.46; I2 = 27%). Side effects included nausea, vomiting, diarrhoea, abdominal cramping, rash, and allergic reaction.Both azithromycin (RR 0.24, 95% CI 0.17 to 0.34; six trials, 374 women) and clindamycin (RR 0.44, 95% CI 0.22 to 0.87; two trials, 183 women) were associated with a lower incidence of side effects compared to erythromycin. These side effects included nausea, vomiting, diarrhoea and abdominal cramping.One small study (101 women) reported there was no clear difference in the number of women with side effects when amoxicillin was compared with clindamycin (RR 0.57, 95% CI 0.14 to 2.26; 107 women). The side effects reported included rash and gastrointestinal complaints. Other secondary outcomes Single trials reported data on repeated infections, preterm birth, preterm rupture of membranes, perinatal mortality and low birthweight and found no clear differences between treatments.Many of this review's secondary outcomes were not reported in the included studies.
Authors' conclusions: Treatment with antibacterial agents achieves microbiological cure from C.trachomatis infection during pregnancy. There was no apparent difference between assessed agents (amoxicillin, erythromycin, clindamycin, azithromycin) in terms of efficacy (microbiological cure and repeat infection) and pregnancy complications (preterm birth, preterm rupture of membranes, low birthweight). Azithromycin and clindamycin appear to result in fewer side effects than erythromycin.All of the studies in this review were conducted in North America, which may limit the generalisability of the results. In addition, study populations may differ in low-resource settings and these results are therefore only applicable to well-resourced settings. Furthermore, the trials in this review mainly took place in the nineties and early 2000's and antibiotic resistance may have changed since then.Further well-designed studies, with appropriate sample sizes and set in a variety of settings, are required to further evaluate interventions for treating C.trachomatis infection in pregnancy and determine which agents achieve the best microbiological cure with the least side effects. Such studies could report on the outcomes listed in this review.
Conflict of interest statement
Natalia Novikova: none known.
Catherine Cluver: none known.
David OA Eriksson: received a small travel scholarship from the international department of Lund University to finance some of the costs for travelling from Sweden to South Africa, to be a part of this review.
Kevin Bengtsson: received a small travel scholarship from the international department of Lund University to finance some of the costs for travelling from Sweden to South Africa, to be a part of this review.
Göran K Lingman: none known.
Figures
Update of
References
References to studies included in this review
Adair 1998 {published data only}
-
- Adair CD, Gunter M, Stovall TG, Mcelroy G, Veille J, Ernest JM. Chlamydia in pregnancy: a randomized trial of azithromycin and erythromycin. Obstetrics & Gynecology 1998;91(2):165‐8. - PubMed
Alary 1994 {published data only}
-
- Alary M, Joly JR, Moutquin JM, Mondor M, Boucher M, Fortier A, et al. Randomised comparison of amoxycillin and erythromycin in treatment of genital chlamydial infection in pregnancy. Lancet 1994;344:1461‐5. - PubMed
Alger 1991 {published data only}
-
- Alger LS, Lovchik JC. Comparative efficacy of clindamycin vs erythromycin in eradication of antenatal chlamydia trachomatis. American Journal of Obstetrics and Gynecology 1991;165:375‐81. - PubMed
Bell 1982 {published data only}
-
- Bell T, Sandstrom I, Eschenbach D, Hummel D, Kuo C, Wang S, et al. Treatment of Chlamydia trachomatis in pregnancy with amoxicillin. Fernstrom Foundation Series 1982;2:221‐4.
Bush 1994 {published data only}
-
- Bush MR, Rosa C. Azithromycin and erythromycin in the treatment of cervical chlamydial infection during pregnancy. Obstetrics & Gynecology 1994;84(1):61‐3. - PubMed
Edwards 1996 {published data only}
Gunter 1996 {published data only}
-
- Gunter ME, Adair CD, Ernest JM, McElroy G. Azithromycin powder versus erthromycin in the treatment of chlamydial cervicitis in pregnancy. Infectious Diseases in Obstetrics and Gynecology 1996;4:53.
Jacobson 2001 {published data only}
-
- Jacobson GF, Autry AM, Kirby RS, Liverman EM, Motley RU. A randomized controlled trial comparing amoxicillin and azithromycin for the treatment of chlamydia trachomatis in pregnancy. American Journal of Obstetrics and Gynecology 2001;184(7):1352‐4; discussion 1354‐6. - PubMed
Kacmar 2001 {published data only}
Magat 1993 {published data only}
-
- Magat AH, Alger LS, Nagey DA, Hatch V, Lovchik JC. Double‐blind randomized study comparing amoxicillin and erythromycin for the treatment of chlamydia trachomatis in pregnancy. Obstetrics & Gynecology 1993;81(5):745‐9. - PubMed
Martin 1997 {published data only}
Rosenn 1995 {published data only}
Silverman 1994 {published data only}
-
- Silverman N, Sullivan M, Hochman M, Womack M, Jungkind DL. A randomized, prospective trial of amoxicillin vs erythromycin for the treatment of chlamydia in pregnancy. American Journal of Obstetrics and Gynecology 1993;168:420. - PubMed
-
- Silverman NS, Sullivan M, Hochman M, Womack M, Jungkind DL. A randomized, prospective trial comparing amoxicillin and erythromycin for the treatment of chlamydia trachomatis in pregnancy. American Journal of Obstetrics and Gynecology 1994;170:829‐32. - PubMed
Turrentine 1995 {published data only}
Wehbeh 1998 {published data only}
-
- Wehbeh H, Ruggiero R, Ali Y, Lopez G, Shahem S, Zarou D. A randomised clinical trial of a single dose of azithromycin in treatment of Chlamydia amongst pregnant women. American Journal of Obstetrics and Gynecology 1996;174(1 Pt 2):361.
-
- Wehbeh HA, Ruggeirio RM, Shahem S, Lopez G, Ali Y. Single‐dose azithromycin for chlamydia in pregnant women. Journal of Reproductive Medicine 1998;43(6):509‐14. - PubMed
References to studies excluded from this review
El‐Shourbagy 2011 {published data only}
-
- El‐Shourbagy MAA, El‐Refaie TA, Sayed KKA, Wahba KAH, El‐Din ASS, Fathy MM. Impact of seroconversion and antichlamydial treatment on the rate of pre‐eclampsia among Egyptian primigravidae. International Journal of Gynecology & Obstetrics 2011;113(2):137‐40. - PubMed
McGregor 1990 {published data only}
-
- McGregor JA, French JI, Richter R, Vuchetich M, Bachus V, Seo K, et al. Cervicovaginal microflora and pregnancy outcome: results of a double‐blind, placebo‐controlled trial of erythromycin treatment. American Journal of Obstetrics and Gynecology 1990;163:1580‐91. - PubMed
Nadafi 2005 {published data only}
-
- Nadafi M, Abdali KH, Parsanejad ME, Rajaee‐Fard AR, Kaviani M. A comparison of amoxicillin and erythromycin for asymptomatic chlamydia trachomatis infection in pregnancy. International Journal of Gynecology & Obstetrics 2005;90(2):142‐3. - PubMed
Zulkarneev 1998 {published data only}
-
- Zulkarneev RS, Kalinin IuT, Afanas'ev SS, Rubal'skii OV, Denisov LA, Vorob'ev AA, et al. Use of recombinant alpha2‐interferon and a complex immunoglobulin preparation for the treatment of chlamydiosis in pregnancy women [Primenenie rekombinantnogo alpha2‐interferona i kompleksnogo immunoglobulinovogo preparata pri lechenii khlamidioza u beremennykh.]. Zhurnal Mikrobiologii, Epidemiologii i Immunobiologii 1998;2:115‐8. - PubMed
References to ongoing studies
Okunola 2013 {published data only}
-
- NCT01946256. Erythromycin versus amoxicillin for treatment of antenatal chlamydia trachomatis infection: a randomized controlled trial. clinicaltrials.gov/ct2/show/NCT01946256 (first received: 16 September 2013).
Additional references
Attenburrow 1985
Berggren 2011
Blas 2007
CDC 2015
-
- Centers for Disease Control and Prevention. 2015 Sexually Transmitted Diseases Treatment Guidelines. Division of STD Prevention, National Center for HIV/AIDS, Viral Hepatitis, STD, and TB Prevention, Centers for Disease Control and Prevention 2015.
Handsfield 2011
-
- Handsfield HH. Questioning azithromycin for chlamydial infection. Sexually Transmitted Diseases 2011;58:1028‐9. - PubMed
Higgins 2011
-
- Higgins JPT, Green S, editors. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Horner 2006
Ismail 1987
-
- Ismail MA, Moawad AH, Poon E, Henderson C. Role of Chlamydia trachomatis in postpartum endometritis. Journal of Reproductive Medicine 1987;32(4):280‐4. - PubMed
Jespersen 2005
Kakar 2010
-
- Kakar S, Bhalla P, Maria A, Rana M, Chawla R, Mathur NB. Chlamydia trachomatis causing neonatal conjunctivitis in a tertiary care centre. Indian Journal of Medical Microbiology 2010;28(1):45‐7. - PubMed
Marrazzo 2016
-
- Marrazzo J. Treatment of Chlamydia trachomatis infection. http://www.uptodate.com/contents/treatment‐of‐chlamydia‐trachomatis‐infe... (accessed Jan 19, 2016) 2016.
Miller 2000
-
- Miller JM, Martin DH. Treatment of Chlamydia trachomatis infections in pregnant women. Drugs 2000;60(3):597‐605. - PubMed
Much 1991
Nigro 2011
-
- Nigro G, Mazzocco M, Mattia E, Renzo GC, Carta G, Anceschi MM. Role of the infections in recurrent spontaneous abortion. Journal of Maternal‐Fetal and Neonatal Medicine 2011;24(8):983‐9. - PubMed
Pammi 2012
-
- Pammi M, Hammerschlag MR. Chlamydia trachomatis infections in the newborn. http://www.uptodate.com/contents/chlamydia‐trachomatis‐infections‐in‐the... (accessed 2012).
Pararas 2006
-
- Pararas MV, Skevaki CL, Kafetzis DA. Preterm birth due to maternal infection: causative pathogens and modes of prevention. European Journal of Clinical Microbiology and Infectious Diseases 2006;25(9):562‐9. - PubMed
RevMan 2014 [Computer program]
-
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.3. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Rours 2009
-
- Rours GI, Hammerschlag MR, Doornum GJ, Hop WC, Groot R, Willemse HF, et al. Chlamydia trachomatis respiratory infection in Dutch infants. Archives of Disease in Childhood 2009;94(9):705‐7. - PubMed
Rours 2011
Schwebke 2011
Silva 2011
-
- Silva MJ, Florêncio GL, Gabiatti JR, Amaral RL, Eleutério Júnior J, Gonçalves AK. Perinatal morbidity and mortality associated with chlamydial infection: a meta‐analysis study. Brazilian Journal of Infectious Diseases 2011;15(6):533‐9. - PubMed
South African STI guideline 2015
-
- sahivsoc.org. Sexually Transmitted Infections Managment Guidelines 2015. http://www.sahivsoc.org/upload/documents/STIguidelines‐1‐28‐15(LC).pdf (accessed 16 May 2016) 2015.
Walker 2012
Workowski 2010
-
- Workowski KA, Berman S. Sexually Transmitted Diseases Treatment Guidelines. CDC, 2010. - PubMed
Yu 2009
-
- Yu J, Wu S, Li F, Hu L. Vertical transmission of Chlamydia trachomatis in Chongqing China. Current Microbiology 2009;58(4):315‐20. - PubMed
Zenilman 2012
-
- Zenilman JM. Genital Chlamydia trachomatis infections in women. http://www.uptodate.com/contents/genital‐chlamydia‐trachomatis‐infection... (accessed 2012).
References to other published versions of this review
Brocklehurst 1998
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical