Advax4 delta inulin combination adjuvant together with ECMX, a fusion construct of four protective mTB antigens, induces a potent Th1 immune response and protects mice against Mycobacterium tuberculosis infection
- PMID: 28937879
- PMCID: PMC5718803
- DOI: 10.1080/21645515.2017.1368598
Advax4 delta inulin combination adjuvant together with ECMX, a fusion construct of four protective mTB antigens, induces a potent Th1 immune response and protects mice against Mycobacterium tuberculosis infection
Abstract
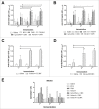
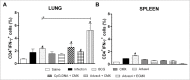
Tuberculosis (TB) remains a main public health concern and 10.4 million new cases occurred in 2015 around the world. BCG is the only approved vaccine against TB, but has variable efficacy and new vaccines are needed. We developed two new mTB vaccine candidates based on the recombinant fusion proteins, rCMX and rECMX formulated with Advax4, a new combination adjuvant combining delta inulin, CpG oligonucleotide and murabutide. BALB/c mice were immunized three times intramuscularly with these vaccine formulations. Injection of Advax4 alone increased the percentage of lymphatic endothelial cells and activated macrophages (F480/CD11b+) in the draining lymph nodes consistent with a chemotactic adjuvant effect. Advax4+CMX and Advax4+ECMX induced the highest levels of IgG1 and IgG2a antibodies against rCMX and rECMX, respectively. Immunized mice challenged with Mycobacterium tuberculosis (Mtb) had increased vaccine-specific Th1 responses in the lungs together with reduced Mtb - associated alveolar damage, although only the Advax4+ECMX vaccine demonstrated significant reduction of lung bacterial load. This study confirmed Advax4+ECMX as a potential TB vaccine candidate, with potential for further optimization and clinical development.
Keywords: ECMX; TB; adjuvant; advax; delta inulin; tuberculosis; vaccine.
Figures




Similar articles
-
A multistage-polyepitope vaccine protects against Mycobacterium tuberculosis infection in HLA-DR3 transgenic mice.Vaccine. 2012 Dec 14;30(52):7513-21. doi: 10.1016/j.vaccine.2012.10.045. Epub 2012 Oct 24. Vaccine. 2012. PMID: 23103299
-
Immunogenicity and therapeutic effects of recombinant Ag85AB fusion protein vaccines in mice infected with Mycobacterium tuberculosis.Vaccine. 2017 Jul 13;35(32):3995-4001. doi: 10.1016/j.vaccine.2017.05.083. Vaccine. 2017. PMID: 28625522
-
Protection of mice from Mycobacterium tuberculosis by ID87/GLA-SE, a novel tuberculosis subunit vaccine candidate.Vaccine. 2011 Oct 13;29(44):7842-8. doi: 10.1016/j.vaccine.2011.07.094. Epub 2011 Aug 2. Vaccine. 2011. PMID: 21816196 Free PMC article.
-
Current and novel approaches to vaccine development against tuberculosis.Front Cell Infect Microbiol. 2012 Dec 6;2:154. doi: 10.3389/fcimb.2012.00154. eCollection 2012. Front Cell Infect Microbiol. 2012. PMID: 23230563 Free PMC article. Review.
-
The current state of tuberculosis vaccines.Hum Vaccin Immunother. 2013 Oct;9(10):2142-6. doi: 10.4161/hv.25427. Epub 2013 Jun 21. Hum Vaccin Immunother. 2013. PMID: 23792698 Free PMC article. Review.
Cited by
-
Polysaccharide PCP-I isolated from Poria cocos enhances the immunogenicity and protection of an anthrax protective antigen-based vaccine.Hum Vaccin Immunother. 2020 Jul 2;16(7):1699-1707. doi: 10.1080/21645515.2019.1675457. Epub 2019 Dec 6. Hum Vaccin Immunother. 2020. PMID: 31809637 Free PMC article.
-
Randomized controlled trial demonstrating the benefits of delta inulin adjuvanted immunotherapy in patients with bee venom allergy.J Allergy Clin Immunol. 2019 Aug;144(2):504-513.e16. doi: 10.1016/j.jaci.2019.03.035. Epub 2019 Jul 9. J Allergy Clin Immunol. 2019. PMID: 31300280 Free PMC article. Clinical Trial.
-
A recombinant Artemisia vulgaris pollen adjuvanted Art v 1 protein-based vaccine treats allergic rhinitis and bronchial asthma using pre- and co-seasonal ultrashort immunotherapy regimens in sensitized mice.Front Immunol. 2022 Nov 9;13:983621. doi: 10.3389/fimmu.2022.983621. eCollection 2022. Front Immunol. 2022. PMID: 36439113 Free PMC article.
-
Evaluation of a Novel Adjuvanted Vaccine for Ultrashort Regimen Therapy of Artemisia Pollen-Induced Allergic Bronchial Asthma in a Mouse Model.Front Immunol. 2022 Mar 15;13:828690. doi: 10.3389/fimmu.2022.828690. eCollection 2022. Front Immunol. 2022. PMID: 35371056 Free PMC article.
-
Protease-Based Subunit Vaccine in Mice Boosts BCG Protection against Mycobacterium tuberculosis.Vaccines (Basel). 2022 Feb 16;10(2):306. doi: 10.3390/vaccines10020306. Vaccines (Basel). 2022. PMID: 35214766 Free PMC article.
References
-
- World Health Organisation Global tuberculosis report 2016. ISBN 9789241565394. Available at: http://www.who.int/tb/publications/global_report/en/
-
- Manabe YC, Bishai WR. Latent Mycobacterium tuberculosis-persistence, patience, and winning by waiting. Nat Med. 2000;6:1327–9. https://doi.org/ 10.1038/82139 PMID:11100115 - DOI - PubMed
-
- Fletcher HA, Schrager L. TB vaccine development and the End TB Strategy: importance and current status. Trans R Soc Trop Med Hyg. 2016;110:212–8. https://doi.org/ 10.1093/trstmh/trw016 PMID:27076508 - DOI - PMC - PubMed
-
- Dockrell HM. Towards new TB vaccines: What are the challenges?. Pathog Dis. 2016;74:ftw016. https://doi.org/ 10.1093/femspd/ftw016 PMID:26960944 - DOI - PubMed
-
- Agger EM. Novel adjuvant formulations for delivery of anti-tuberculosis vaccine candidates. Adv Drug Deliv Rev. 2016;1:73–82. https://doi.org/ 10.1016/j.addr.2015.11.012 - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
