A new discrete dynamic model of ABA-induced stomatal closure predicts key feedback loops
- PMID: 28937978
- PMCID: PMC5627951
- DOI: 10.1371/journal.pbio.2003451
A new discrete dynamic model of ABA-induced stomatal closure predicts key feedback loops
Update in
-
A network-based modeling framework reveals the core signal transduction network underlying high carbon dioxide-induced stomatal closure in guard cells.PLoS Biol. 2024 May 1;22(5):e3002592. doi: 10.1371/journal.pbio.3002592. eCollection 2024 May. PLoS Biol. 2024. PMID: 38691548 Free PMC article.
Abstract
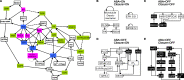
Stomata, microscopic pores in leaf surfaces through which water loss and carbon dioxide uptake occur, are closed in response to drought by the phytohormone abscisic acid (ABA). This process is vital for drought tolerance and has been the topic of extensive experimental investigation in the last decades. Although a core signaling chain has been elucidated consisting of ABA binding to receptors, which alleviates negative regulation by protein phosphatases 2C (PP2Cs) of the protein kinase OPEN STOMATA 1 (OST1) and ultimately results in activation of anion channels, osmotic water loss, and stomatal closure, over 70 additional components have been identified, yet their relationships with each other and the core components are poorly elucidated. We integrated and processed hundreds of disparate observations regarding ABA signal transduction responses underlying stomatal closure into a network of 84 nodes and 156 edges and, as a result, established those relationships, including identification of a 36-node, strongly connected (feedback-rich) component as well as its in- and out-components. The network's domination by a feedback-rich component may reflect a general feature of rapid signaling events. We developed a discrete dynamic model of this network and elucidated the effects of ABA plus knockout or constitutive activity of 79 nodes on both the outcome of the system (closure) and the status of all internal nodes. The model, with more than 1024 system states, is far from fully determined by the available data, yet model results agree with existing experiments in 82 cases and disagree in only 17 cases, a validation rate of 75%. Our results reveal nodes that could be engineered to impact stomatal closure in a controlled fashion and also provide over 140 novel predictions for which experimental data are currently lacking. Noting the paucity of wet-bench data regarding combinatorial effects of ABA and internal node activation, we experimentally confirmed several predictions of the model with regard to reactive oxygen species, cytosolic Ca2+ (Ca2+c), and heterotrimeric G-protein signaling. We analyzed dynamics-determining positive and negative feedback loops, thereby elucidating the attractor (dynamic behavior) repertoire of the system and the groups of nodes that determine each attractor. Based on this analysis, we predict the likely presence of a previously unrecognized feedback mechanism dependent on Ca2+c. This mechanism would provide model agreement with 10 additional experimental observations, for a validation rate of 85%. Our research underscores the importance of feedback regulation in generating robust and adaptable biological responses. The high validation rate of our model illustrates the advantages of discrete dynamic modeling for complex, nonlinear systems common in biology.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures





References
-
- Assmann SM, Jegla T. Guard cell sensory systems: recent insights on stomatal responses to light, abscisic acid, and CO2. Curr Opin Plant Biol. 2016;33:157–67. Epub 2016/08/16. doi: 10.1016/j.pbi.2016.07.003 . - DOI - PubMed
-
- Joshi-Saha A, Valon C, Leung J. Abscisic acid signal off the STARting block. Mol Plant. 2011;4(4):562–80. doi: 10.1093/mp/ssr055 . - DOI - PubMed
-
- Munemasa S, Hauser F, Park J, Waadt R, Brandt B, Schroeder JI. Mechanisms of abscisic acid-mediated control of stomatal aperture. Curr Opin Plant Biol. 2015;28:154–62. Epub 2015/11/26. doi: 10.1016/j.pbi.2015.10.010 ; PubMed Central PMCID: PMC4679528. - DOI - PMC - PubMed
-
- Kim TH, Bohmer M, Hu H, Nishimura N, Schroeder JI. Guard cell signal transduction network: advances in understanding abscisic acid, CO2, and Ca2+ signaling. Annu Rev Plant Biol. 2010;61:561–91. Epub 2010/03/03. doi: 10.1146/annurev-arplant-042809-112226 ; PubMed Central PMCID: PMC3056615. - DOI - PMC - PubMed
-
- Acharya BR, Assmann SM. Hormone interactions in stomatal function. Plant Mol Biol. 2009;69(4):451–62. doi: 10.1007/s11103-008-9427-0 . - DOI - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

