SIRT1 is a positive regulator of in vivo bone mass and a therapeutic target for osteoporosis
- PMID: 28937996
- PMCID: PMC5609767
- DOI: 10.1371/journal.pone.0185236
SIRT1 is a positive regulator of in vivo bone mass and a therapeutic target for osteoporosis
Abstract
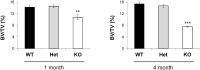
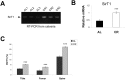
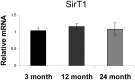
Overexpression or pharmacological activation of SIRT1 has been shown to extend the lifespan of mice and protect against aging-related diseases. Here we show that pharmacological activation of SIRT1 protects in two models of osteoporosis. Ovariectomized female mice and aged male mice, models for post-menopausal and aging-related osteoporosis, respectively, show significant improvements in bone mass upon treatment with SIRT1 agonist, SRT1720. Further, we find that calorie restriction (CR) results in a two-fold upregulation of sirt1 mRNA expression in bone tissue that is associated with increased bone mass in CR mice. Reciprocally, SIRT1 whole-body knockout (KO) mice, as well as osteoblast and osteoclast specific KOs, show a low bone mass phenotype; though double knockout mice (containing SIRT1 deleted in both osteoblasts and osteoclasts) do not show a more severe phenotype. Altogether, these findings provide strong evidence that SIRT1 is a positive regulator of bone mass and a promising target for the development of novel therapeutics for osteoporosis.
Conflict of interest statement
Figures



References
-
- Haigis MC, Guarente LP. Mammalian sirtuins—emerging roles in physiology aging and calorie restriction. Genes Dev. 2006;20:2913–21. doi: 10.1101/gad.1467506 - DOI - PubMed
-
- Imai S, Armstrong CM, Kaeberlein M, Guarente L. Transcriptional silencing and longevity protein Sir2 is an NAD-dependent histone deacetylase. Nature. 2000;403:795–800. doi: 10.1038/35001622 - DOI - PubMed
-
- Kennedy BK, Austriaco NR Jr, Zhang J, Guarente L. Mutation in the silencing gene SIR4 can delay aging in S. cerevisiae. Cell. 1995;80:485–96. - PubMed
-
- Kennedy BK, Gotta M, Sinclair DA, Mills K, McNabb DS, Murthy M, et al. Redistribution of silencing proteins from telomeres to the nucleolus is associated with extension of life span in S. cerevisiae. Cell. 1997;8:381–91. - PubMed
-
- Tissenbaum HA, Guarente L. Increased dosage of a sir-2 gene extends lifespan in Caenorhabditis elegans. Nature. 2001;410:227–30. doi: 10.1038/35065638 - DOI - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

