Recombinant vaccines of a CD4+ T-cell epitope promote efficient control of Paracoccidioides brasiliensis burden by restraining primary organ infection
- PMID: 28938005
- PMCID: PMC5627964
- DOI: 10.1371/journal.pntd.0005927
Recombinant vaccines of a CD4+ T-cell epitope promote efficient control of Paracoccidioides brasiliensis burden by restraining primary organ infection
Abstract
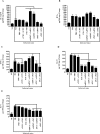
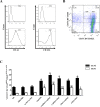
Paracoccidioidomycosis (PCM) is an infectious disease endemic to South America, caused by the thermally dimorphic fungi Paracoccidioides. Currently, there is no effective human vaccine that can be used in prophylactic or therapeutic regimes. We tested the hypothesis that the immunogenicity of the immunodominant CD4+ T-cell epitope (P10) of Paracoccidioides brasiliensis gp43 antigen might be significantly enhanced by using a hepatitis B virus-derived particle (VLP) as an antigen carrier. This chimera was administered to mice as a (His)6-purified protein (rPbT) or a replication-deficient human type 5 adenoviral vector (rAdPbT) in an immunoprophylaxis assay. The highly virulent Pb18 yeast strain was used to challenge our vaccine candidates. Fungal challenge evoked robust P10-specific memory CD4+ T cells secreting protective Th-1 cytokines in most groups of immunized mice. Furthermore, the highest level of fungal burden control was achieved when rAdPbT was inoculated in a homologous prime-boost regimen, with 10-fold less CFU recovering than in non-vaccinated mice. Systemic Pb18 spreading was only prevented when rAdPbT was previously inoculated. In summary, we present here VLP/P10 formulations as vaccine candidates against PCM, some of which have demonstrated for the first time their ability to prevent progression of this pernicious fungal disease, which represents a significant social burden in developing countries.
Conflict of interest statement
I have read the journal's policy and the authors of this manuscript have the following competing interests: RAH, DAS, CPT and OBR are inventors on patent application [BR1020150311150] of the recombinant vaccines that was assigned by National Institute of Industrial Property, Brazil.
Figures






References
-
- Franco M, Bagagli E, Scapolio S, da Silva Lacaz C. A critical analysis of isolation of Paracoccidioides brasiliensis from soil. Med Mycol 2000; 38:185–91. - PubMed
-
- Bocca AL, Amaral AC, Teixeira MM, Sato PK, Shikanai-Yasuda MA, Soares Felipe MS. Paracoccidioidomycosis: eco-epidemiology, taxonomy and clinical and therapeutic issues. Future Microbiol 2013; 8:1177–91. doi: 10.2217/fmb.13.68 - DOI - PubMed
-
- Stevens DA, Brummer E, Clemons KV. Interferon- gamma as an antifungal. The J Infect Dis 2006; 194 Suppl 1:S33–7. - PubMed
-
- Iwai LK, Yoshida M, Sadahiro A, da Silva WR, Marin ML, Goldberg AC, et al. T-cell recognition of Paracoccidioides brasiliensis gp43-derived peptides in patients with paracoccidioidomycosis and healthy individuals. Clin Vaccine Immunol 2007; 14:474–6. doi: 10.1128/CVI.00458-06 - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

