VCA nanobodies target N-WASp to reduce invadopodium formation and functioning
- PMID: 28938008
- PMCID: PMC5609757
- DOI: 10.1371/journal.pone.0185076
VCA nanobodies target N-WASp to reduce invadopodium formation and functioning
Abstract
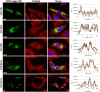
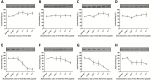
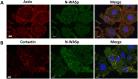
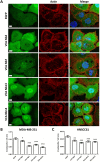
Invasive cancer cells develop small actin-based protrusions called invadopodia, which perform a primordial role in metastasis and extracellular matrix remodelling. Neural Wiskott-Aldrich syndrome protein (N-WASp) is a scaffold protein which can directly bind to actin monomers and Arp2/3 and is a crucial player in the formation of an invadopodium precursor. Expression modulation has pointed to an important role for N-WASp in invadopodium formation but the role of its C-terminal VCA domain in this process remains unknown. In this study, we generated alpaca nanobodies against the N-WASp VCA domain and investigated if these nanobodies affect invadopodium formation. By using this approach, we were able to study functions of a selected functional/structural N-WASp protein domain in living cells, without requiring overexpression, dominant negative mutants or siRNAs which target the gene, and hence the entire protein. When expressed as intrabodies, the VCA nanobodies significantly reduced invadopodium formation in both MDA-MB-231 breast cancer and HNSCC61 head and neck squamous cancer cells. Furthermore, expression of distinct VCA Nbs (VCA Nb7 and VCA Nb14) in PC-3 prostate cancer cells resulted in reduced overall matrix degradation without affecting MMP9 secretion/activation or MT1-MMP localisation at invadopodial membranes. From these results, we conclude that we have generated nanobodies targeting N-WASp which reduce invadopodium formation and functioning, most likely via regulation of N-WASp-Arp2/3 complex interaction, indicating that this region of N-WASp plays an important role in these processes.
Conflict of interest statement
Figures

Similar articles
-
Nanobodies targeting cortactin proline rich, helical and actin binding regions downregulate invadopodium formation and matrix degradation in SCC-61 cancer cells.Biomed Pharmacother. 2018 Jun;102:230-241. doi: 10.1016/j.biopha.2018.03.064. Epub 2018 Mar 22. Biomed Pharmacother. 2018. PMID: 29567535
-
Stratifying fascin and cortactin function in invadopodium formation using inhibitory nanobodies and targeted subcellular delocalization.FASEB J. 2014 Apr;28(4):1805-18. doi: 10.1096/fj.13-242537. Epub 2014 Jan 10. FASEB J. 2014. PMID: 24414419
-
Interactions of isolated C-terminal fragments of neural Wiskott-Aldrich syndrome protein (N-WASP) with actin and Arp2/3 complex.J Biol Chem. 2012 Oct 5;287(41):34646-59. doi: 10.1074/jbc.M112.394361. Epub 2012 Jul 30. J Biol Chem. 2012. PMID: 22847007 Free PMC article.
-
The 'ins' and 'outs' of podosomes and invadopodia: characteristics, formation and function.Nat Rev Mol Cell Biol. 2011 Jun 23;12(7):413-26. doi: 10.1038/nrm3141. Nat Rev Mol Cell Biol. 2011. PMID: 21697900 Free PMC article. Review.
-
Digging a little deeper: the stages of invadopodium formation and maturation.Eur J Cell Biol. 2014 Oct;93(10-12):438-44. doi: 10.1016/j.ejcb.2014.07.003. Epub 2014 Jul 21. Eur J Cell Biol. 2014. PMID: 25113547 Free PMC article. Review.
Cited by
-
Role of actin-binding proteins in prostate cancer.Front Cell Dev Biol. 2024 Jul 11;12:1430386. doi: 10.3389/fcell.2024.1430386. eCollection 2024. Front Cell Dev Biol. 2024. PMID: 39055653 Free PMC article. Review.
-
Nanobody-Based Probes for Subcellular Protein Identification and Visualization.Front Cell Neurosci. 2020 Nov 2;14:573278. doi: 10.3389/fncel.2020.573278. eCollection 2020. Front Cell Neurosci. 2020. PMID: 33240044 Free PMC article. Review.
-
WASP family proteins: Molecular mechanisms and implications in human disease.Eur J Cell Biol. 2022 Jun-Aug;101(3):151244. doi: 10.1016/j.ejcb.2022.151244. Epub 2022 Jun 1. Eur J Cell Biol. 2022. PMID: 35667337 Free PMC article.
-
Nanobodies Right in the Middle: Intrabodies as Toolbox to Visualize and Modulate Antigens in the Living Cell.Biomolecules. 2020 Dec 21;10(12):1701. doi: 10.3390/biom10121701. Biomolecules. 2020. PMID: 33371447 Free PMC article. Review.
-
Applying Antibodies Inside Cells: Principles and Recent Advances in Neurobiology, Virology and Oncology.BioDrugs. 2020 Aug;34(4):435-462. doi: 10.1007/s40259-020-00419-w. BioDrugs. 2020. PMID: 32301049 Free PMC article. Review.
References
-
- Kim MY, Oskarsson T, Acharyya S, Nguyen DX, Zhang XHF, Norton L, et al. Tumor Self-Seeding by Circulating Cancer Cells. Cell. 2009;139(7):1315–26. doi: 10.1016/j.cell.2009.11.025 - DOI - PMC - PubMed
-
- Stylli SS, Kaye AH, Lock P. Invadopodia: At the cutting edge of tumour invasion. Journal of Clinical Neuroscience. 2008;15(7):725–37. doi: 10.1016/j.jocn.2008.03.003 - DOI - PubMed
-
- Leong HS, Robertson AE, Stoletov K, Leith SJ, Chin CA, Chien AE, et al. Invadopodia Are Required for Cancer Cell Extravasation and Are a Therapeutic Target for Metastasis. Cell Reports. 2014;8(5):1558–70. doi: 10.1016/j.celrep.2014.07.050 - DOI - PubMed
-
- Yamaguchi H, Wyckoff J, Condeelis J. Cell migration in tumors. Current Opinion in Cell Biology. 2005;17(5):559–64. http://dx.doi.org/10.1016/j.ceb.2005.08.002. - DOI - PubMed
-
- Siar CH, Rahman ZABA, Tsujigiwa H, Mohamed Om Alblazi K, Nagatsuka H, Ng KH. Invadopodia proteins, cortactin, N-WASP and WIP differentially promote local invasiveness in ameloblastoma. Journal of Oral Pathology & Medicine. 2016. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

