Intestinal helminth infection drives carcinogenesis in colitis-associated colon cancer
- PMID: 28938014
- PMCID: PMC5627963
- DOI: 10.1371/journal.ppat.1006649
Intestinal helminth infection drives carcinogenesis in colitis-associated colon cancer
Abstract
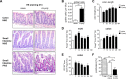
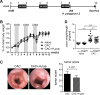
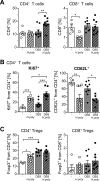
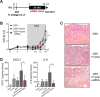
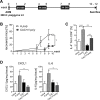
Inflammatory bowel diseases (IBD) are chronic inflammatory disorders of the gastrointestinal tract, strongly associated with an increased risk of colorectal cancer development. Parasitic infections caused by helminths have been shown to modulate the host's immune response by releasing immunomodulatory molecules and inducing regulatory T cells (Tregs). This immunosuppressive state provoked in the host has been considered as a novel and promising approach to treat IBD patients and alleviate acute intestinal inflammation. On the contrary, specific parasite infections are well known to be directly linked to carcinogenesis. Whether a helminth infection interferes with the development of colitis-associated colon cancer (CAC) is not yet known. In the present study, we demonstrate that the treatment of mice with the intestinal helminth Heligmosomoides polygyrus at the onset of tumor progression in a mouse model of CAC does not alter tumor growth and distribution. In contrast, H. polygyrus infection in the early inflammatory phase of CAC strengthens the inflammatory response and significantly boosts tumor development. Here, H. polygyrus infection was accompanied by long-lasting alterations in the colonic immune cell compartment, with reduced frequencies of colonic CD8+ effector T cells. Moreover, H. polygyrus infection in the course of dextran sulfate sodium (DSS) mediated colitis significantly exacerbates intestinal inflammation by amplifying the release of colonic IL-6 and CXCL1. Thus, our findings indicate that the therapeutic application of helminths during CAC might have tumor-promoting effects and therefore should be well-considered.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures
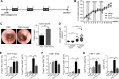

References
-
- Elinav E, Nowarski R, Thaiss CA, Hu B, Jin C, Flavell RA. Inflammation-induced cancer: crosstalk between tumours, immune cells and microorganisms. Nat Rev Cancer. 2013;13(11):759–71. doi: 10.1038/nrc3611 - DOI - PubMed
-
- Axelrad JE, Lichtiger S, Yajnik V. Inflammatory bowel disease and cancer: The role of inflammation, immunosuppression, and cancer treatment. World J Gastroenterol. 2016;22(20):4794–801. doi: 10.3748/wjg.v22.i20.4794 - DOI - PMC - PubMed
-
- Baumgart DC, Carding SR. Inflammatory bowel disease: cause and immunobiology. Lancet. 2007;369(9573):1627–40. doi: 10.1016/S0140-6736(07)60750-8 - DOI - PubMed
-
- Pedros C, Duguet F, Saoudi A, Chabod M. Disrupted regulatory T cell homeostasis in inflammatory bowel diseases. World J Gastroenterol. 2016;22(3):974–95. doi: 10.3748/wjg.v22.i3.974 - DOI - PMC - PubMed
-
- Pastille E, Bardini K, Fleissner D, Adamczyk A, Frede A, Wadwa M, et al. Transient ablation of regulatory T cells improves antitumor immunity in colitis-associated colon cancer. Cancer Res. 2014;74(16):4258–69. doi: 10.1158/0008-5472.CAN-13-3065 - DOI - PubMed
MeSH terms
Supplementary concepts
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

