A feed-forward regulation of endothelin receptors by c-Jun in human non-pigmented ciliary epithelial cells and retinal ganglion cells
- PMID: 28938016
- PMCID: PMC5609771
- DOI: 10.1371/journal.pone.0185390
A feed-forward regulation of endothelin receptors by c-Jun in human non-pigmented ciliary epithelial cells and retinal ganglion cells
Abstract
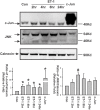
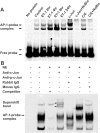
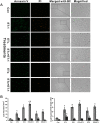
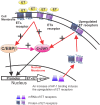
c-Jun, c-Jun N-terminal kinase(JNK) and endothelin B (ETB) receptor have been shown to contribute to the pathogenesis of glaucoma. Previously, we reported that an increase of c-Jun and CCAAT/enhancer binding protein β (C/EBPβ) immunohistostaining is associated with upregulation of the ETB receptor within the ganglion cell layer of rats with elevated intraocular pressure (IOP). In addition, both transcription factors regulate the expression of the ETB receptor in human non-pigmented ciliary epithelial cells (HNPE). The current study addressed the mechanisms by which ET-1 produced upregulation of ET receptors in primary rat retinal ganglion cells (RGCs) and HNPE cells. Treatment of ET-1 and ET-3 increased the immunocytochemical staining of c-Jun and C/EBPβ in primary rat RGCs and co-localization of both transcription factors was observed. A marked increase in DNA binding activity of AP-1 and C/EBPβ as well as elevated protein levels of c-Jun and c-Jun-N-terminal kinase (JNK) were detected following ET-1 treatment in HNPE cells. Overexpression of ETA or ETB receptor promoted the upregulation of c-Jun and also elevated its promoter activity. In addition, upregulation of C/EBPβ augmented DNA binding and mRNA expression of c-Jun, and furthermore, the interaction of c-Jun and C/EBPβ was confirmed using co-immunoprecipitation. Apoptosis of HNPE cells was identified following ET-1 treatment, and overexpression of the ETA or ETB receptor produced enhanced apoptosis. ET-1 mediated upregulation of c-Jun and C/EBPβ and their interaction may represent a novel mechanism contributing to the regulation of endothelin receptor expression. Reciprocally, c-Jun was also found to regulate the ET receptors and C/EBPβ appeared to play a regulatory role in promoting expression of c-Jun. Taken together, the data suggests that ET-1 triggers the upregulation of c-Jun through both ETA and ETB receptors, and conversely c-Jun also upregulates endothelin receptor expression, thereby generating a positive feed-forward loop of endothelin receptor activation and expression. This feed-forward regulation may contribute to RGC death and astrocyte proliferation following ET-1 treatment.
Conflict of interest statement
Figures




Similar articles
-
Involvement of AP-1 and C/EBPβ in upregulation of endothelin B (ETB) receptor expression in a rodent model of glaucoma.PLoS One. 2013 Nov 12;8(11):e79183. doi: 10.1371/journal.pone.0079183. eCollection 2013. PLoS One. 2013. PMID: 24265756 Free PMC article.
-
Role of the ETB receptor in retinal ganglion cell death in glaucoma.Can J Physiol Pharmacol. 2008 Jun;86(6):380-93. doi: 10.1139/Y08-040. Can J Physiol Pharmacol. 2008. PMID: 18516102
-
Upregulation of the endothelin A (ETA) receptor and its association with neurodegeneration in a rodent model of glaucoma.BMC Neurosci. 2017 Mar 1;18(1):27. doi: 10.1186/s12868-017-0346-3. BMC Neurosci. 2017. PMID: 28249604 Free PMC article.
-
Constitutive ALK5-independent c-Jun N-terminal kinase activation contributes to endothelin-1 overexpression in pulmonary fibrosis: evidence of an autocrine endothelin loop operating through the endothelin A and B receptors.Mol Cell Biol. 2006 Jul;26(14):5518-27. doi: 10.1128/MCB.00625-06. Mol Cell Biol. 2006. PMID: 16809784 Free PMC article.
-
Dexamethasone regulates endothelin-1 and endothelin receptors in human non-pigmented ciliary epithelial (HNPE) cells.Exp Eye Res. 2003 Mar;76(3):261-72. doi: 10.1016/s0014-4835(02)00323-8. Exp Eye Res. 2003. PMID: 12573655
Cited by
-
Regulatory mechanisms of retinal ganglion cell death in normal tension glaucoma and potential therapies.Neural Regen Res. 2023 Jan;18(1):87-93. doi: 10.4103/1673-5374.344831. Neural Regen Res. 2023. PMID: 35799514 Free PMC article. Review.
-
Endothelin 1-induced retinal ganglion cell death is largely mediated by JUN activation.Cell Death Dis. 2020 Sep 26;11(9):811. doi: 10.1038/s41419-020-02990-0. Cell Death Dis. 2020. PMID: 32980857 Free PMC article.
-
Functional interaction between endothelin-1 and ZEB1/YAP signaling regulates cellular plasticity and metastasis in high-grade serous ovarian cancer.J Exp Clin Cancer Res. 2022 Apr 28;41(1):157. doi: 10.1186/s13046-022-02317-1. J Exp Clin Cancer Res. 2022. PMID: 35477522 Free PMC article.
-
Involvement of c-Jun N-terminal kinase 2 (JNK2) in Endothelin-1 (ET-1) Mediated Neurodegeneration of Retinal Ganglion Cells.Invest Ophthalmol Vis Sci. 2021 May 3;62(6):13. doi: 10.1167/iovs.62.6.13. Invest Ophthalmol Vis Sci. 2021. PMID: 33978676 Free PMC article.
References
-
- Tham Y-C, Li X, Wong TY, Quigley HA, Aung T, Cheng C-Y. Global Prevalence of Glaucoma and Projections of Glaucoma Burden through 2040: A Systematic Review and Meta-Analysis. Ophthalmology. 2014;121(11):2081–90. doi: 10.1016/j.ophtha.2014.05.013 - DOI - PubMed
-
- Noske, Whensen J, Wiederholt M. Endothelin-like immunoreactivity in aqueous humor of patients with primary open-angle glaucoma and cataract. Graefes Arch Clin Exp Ophthalmol. 1997;235(9):551–2. - PubMed
-
- Tezel G, Kass M, Kolker A, Becker B, Wax M. Plasma and aqueous humor endothelin levels in primary open-angle glaucoma. J Glaucoma. 1997;6(2):83–9. - PubMed
-
- Kallberg ME, Brooks DE, Garcia-Sanchez GA, Komaromy AM, Szabo NJ, Tian L. Endothelin 1 Levels in the Aqueous Humor of Dogs With Glaucoma. J Glaucoma. 2002;11(2):105–9. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

