IDH2 deficiency increases the liver susceptibility to ischemia-reperfusion injury via increased mitochondrial oxidative injury
- PMID: 28938192
- PMCID: PMC5608561
- DOI: 10.1016/j.redox.2017.09.003
IDH2 deficiency increases the liver susceptibility to ischemia-reperfusion injury via increased mitochondrial oxidative injury
Abstract
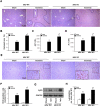
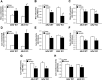
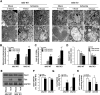
Mitochondrial NADP+-dependent isocitrate dehydrogenase 2 (IDH2) is a major producer of mitochondrial NADPH, required for glutathione (GSH)-associated mitochondrial antioxidant systems including glutathione peroxidase (GPx) and glutathione reductase (GR). Here, we investigated the role of IDH2 in hepatic ischemia-reperfusion (HIR)-associated mitochondrial injury using Idh2-knockout (Idh2-/-) mice and wild-type (Idh2+/+) littermates. Mice were subjected to either 60min of partial liver ischemia or sham-operation. Some mice were administered with 2-(2,2,6,6-tetramethylpiperidin-1-oxyl-4-ylamino)-2-oxoethyl) triphenylphosphonium chloride (mito-TEMPO, a mitochondria-targeting antioxidant). HIR induced severe histological and functional damages of liver in both Idh2+/+ mice and Idh2-/- mice and those damages were more severe in Idh2-/- mice than in wild-type littermates. HIR induces dysfunction of IDH2, leading to the decreases of NADPH level and mitochondrial GR and GPx functions, consequently resulting in mitochondrial and cellular oxidative injury as reflected by mitochondrial cristae loss, mitochondrial fragmentation, shift in mitochondrial fission, cytochrome c release, and cell death. These HIR-induced changes were greater in Idh2-/- mice than wild-type mice. The mito-TEMPO supplement significantly attenuated the aforementioned changes, and these attenuations were much greater in Idh2-/- mice when compared with wild-type littermates. Taken together, results have demonstrated that HIR impairs in the IDH2-NADPH-GSH mitochondrial antioxidant system, resulting in increased mitochondrial oxidative damage and dysfunction, suggesting that IDH2 plays a critical role in mitochondrial redox balance and HIR-induced impairment of IDH2 function is associated with the pathogenesis of ischemia-reperfusion-induced liver failure.
Keywords: Apoptosis; IDH2; Liver ischemia; Mitochondria; Oxidative stress.
Copyright © 2017 The Authors. Published by Elsevier B.V. All rights reserved.
Figures
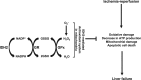





Similar articles
-
Mitochondrial NADP+-dependent isocitrate dehydrogenase deficiency increases cisplatin-induced oxidative damage in the kidney tubule cells.Cell Death Dis. 2018 May 1;9(5):488. doi: 10.1038/s41419-018-0537-6. Cell Death Dis. 2018. PMID: 29695796 Free PMC article.
-
IDH2 deficiency exacerbates acetaminophen hepatotoxicity in mice via mitochondrial dysfunction-induced apoptosis.Biochim Biophys Acta Mol Basis Dis. 2019 Sep 1;1865(9):2333-2341. doi: 10.1016/j.bbadis.2019.05.012. Epub 2019 May 20. Biochim Biophys Acta Mol Basis Dis. 2019. PMID: 31121248
-
Mitochondrial NADP+-Dependent Isocitrate Dehydrogenase Deficiency Exacerbates Mitochondrial and Cell Damage after Kidney Ischemia-Reperfusion Injury.J Am Soc Nephrol. 2017 Apr;28(4):1200-1215. doi: 10.1681/ASN.2016030349. Epub 2016 Nov 7. J Am Soc Nephrol. 2017. PMID: 27821630 Free PMC article.
-
The roles of NADPH and isocitrate dehydrogenase in cochlear mitochondrial antioxidant defense and aging.Hear Res. 2023 Jan;427:108659. doi: 10.1016/j.heares.2022.108659. Epub 2022 Nov 24. Hear Res. 2023. PMID: 36493529 Free PMC article. Review.
-
Mitochondrial glutathione, a key survival antioxidant.Antioxid Redox Signal. 2009 Nov;11(11):2685-700. doi: 10.1089/ARS.2009.2695. Antioxid Redox Signal. 2009. PMID: 19558212 Free PMC article. Review.
Cited by
-
Crosstalk Between Mitochondrial Hyperacetylation and Oxidative Stress in Vascular Dysfunction and Hypertension.Antioxid Redox Signal. 2019 Oct 1;31(10):710-721. doi: 10.1089/ars.2018.7632. Epub 2019 Feb 28. Antioxid Redox Signal. 2019. PMID: 30618267 Free PMC article.
-
Irisin activates Opa1-induced mitophagy to protect cardiomyocytes against apoptosis following myocardial infarction.Aging (Albany NY). 2020 Mar 10;12(5):4474-4488. doi: 10.18632/aging.102899. Epub 2020 Mar 10. Aging (Albany NY). 2020. PMID: 32155590 Free PMC article.
-
Reactive oxygen species-mediated senescence is accelerated by inhibiting Cdk2 in Idh2-deficient conditions.Aging (Albany NY). 2019 Sep 10;11(17):7242-7256. doi: 10.18632/aging.102259. Epub 2019 Sep 10. Aging (Albany NY). 2019. PMID: 31503005 Free PMC article.
-
SIRT5-Related Desuccinylation Modification Contributes to Quercetin-Induced Protection against Heart Failure and High-Glucose-Prompted Cardiomyocytes Injured through Regulation of Mitochondrial Quality Surveillance.Oxid Med Cell Longev. 2021 Sep 23;2021:5876841. doi: 10.1155/2021/5876841. eCollection 2021. Oxid Med Cell Longev. 2021. PMID: 34603599 Free PMC article.
-
IDH2 gene deficiency accelerates unilateral ureteral obstruction-induced kidney inflammation through oxidative stress and activation of macrophages.Korean J Physiol Pharmacol. 2021 Mar 1;25(2):139-146. doi: 10.4196/kjpp.2021.25.2.139. Korean J Physiol Pharmacol. 2021. PMID: 33602884 Free PMC article.
References
-
- Elias-Miro M., Jimenez-Castro M.B., Rodes J., Peralta C. Current knowledge on oxidative stress in hepatic ischemia/reperfusion. Free Radic. Res. 2013;47:555–568. - PubMed
-
- Zhang W., Wang M., Xie H.Y., Zhou L., Meng X.Q., Shi J. Role of reactive oxygen species in mediating hepatic ischemia-reperfusion injury and its therapeutic applications in liver transplantation. Transplant. Proc. 2007;39:1332–1337. - PubMed
-
- Zeng S., Lin Y., Di J.F., Feng Z. Protective effect of N-acetylcysteine on liver and lung in mice after ischemia-reperfusion injury. Xi Bao Yu Fen Zi Mian Yi Xue Za Zhi. 2009;25:1058–1060. - PubMed
-
- Mukhopadhyay P., Horvath B., Zsengeller Z., Batkai S., Cao Z., Kechrid M. Mitochondrial reactive oxygen species generation triggers inflammatory response and tissue injury associated with hepatic ischemia-reperfusion: therapeutic potential of mitochondrially targeted antioxidants. Free Radic. Biol. Med. 2012;53:1123–1138. - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

