IL-1β Inhibits Connexin 43 and Disrupts Decidualization of Human Endometrial Stromal Cells Through ERK1/2 and p38 MAP Kinase
- PMID: 28938400
- PMCID: PMC5711380
- DOI: 10.1210/en.2017-00495
IL-1β Inhibits Connexin 43 and Disrupts Decidualization of Human Endometrial Stromal Cells Through ERK1/2 and p38 MAP Kinase
Abstract
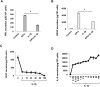
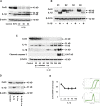
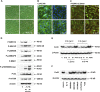
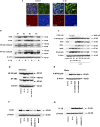
Inflammation can interfere with endometrial receptivity. We examined how interleukin 1β (IL-1β) affects expression of the uterine gap junction protein connexin 43 (Cx43), which is known to be critical for embryonic implantation. We used an in vitro model of human endometrial stromal cells (ESCs), Western blotting, and a combination of validated, selective kinase inhibitors to evaluate five canonical IL-1β signaling pathways. Cx43 and two other markers of ESC differentiation (prolactin and VEGF) were inhibited predominantly via IL-1β-activated ERK1/2 and p38 MAP kinase cascades. The findings were corroborated using small interfering RNA to silence critical genes in either pathway. By contrast, upregulation of endogenous pro-IL-1α and pro-IL-1β following recombinant IL-1β treatment was mediated via the Jun N-terminal kinase pathway. The clinicopharmacological significance of our findings is that multiple signaling cascades may need to be neutralized to reverse deleterious effects of IL-1β on human endometrial function.
Copyright © 2017 Endocrine Society.
Figures



Similar articles
-
hCG activates Epac-Erk1/2 signaling regulating Progesterone Receptor expression and function in human endometrial stromal cells.Mol Hum Reprod. 2017 Jun 1;23(6):393-405. doi: 10.1093/molehr/gax015. Mol Hum Reprod. 2017. PMID: 28333280
-
Disruption of gap junctions reduces biomarkers of decidualization and angiogenesis and increases inflammatory mediators in human endometrial stromal cell cultures.Mol Cell Endocrinol. 2011 Sep 15;344(1-2):25-34. doi: 10.1016/j.mce.2011.04.011. Epub 2011 Jul 8. Mol Cell Endocrinol. 2011. PMID: 21767601 Free PMC article.
-
Interleukin-1beta elevates cyclooxygenase-2 protein level and enzyme activity via increasing its mRNA stability in human endometrial stromal cells: an effect mediated by extracellularly regulated kinases 1 and 2.J Clin Endocrinol Metab. 2002 Jul;87(7):3263-73. doi: 10.1210/jcem.87.7.8594. J Clin Endocrinol Metab. 2002. PMID: 12107235
-
Interleukin-1β inhibits estrogen receptor-α, progesterone receptors A and B and biomarkers of human endometrial stromal cell differentiation: implications for endometriosis.Mol Hum Reprod. 2019 Oct 28;25(10):625-637. doi: 10.1093/molehr/gaz045. Mol Hum Reprod. 2019. PMID: 31408162 Free PMC article.
-
Differentiation of human umbilical cord Wharton's jelly-derived mesenchymal stem cells into endometrial cells.Stem Cell Res Ther. 2017 Nov 2;8(1):246. doi: 10.1186/s13287-017-0700-5. Stem Cell Res Ther. 2017. PMID: 29096715 Free PMC article.
Cited by
-
Towards a Better Understanding of Endometriosis-Related Infertility: A Review on How Endometriosis Affects Endometrial Receptivity.Biomolecules. 2023 Feb 24;13(3):430. doi: 10.3390/biom13030430. Biomolecules. 2023. PMID: 36979365 Free PMC article. Review.
-
Interleukin-1β induces and accelerates human endometrial stromal cell senescence and impairs decidualization via the c-Jun N-terminal kinase pathway.Cell Death Discov. 2024 Jun 15;10(1):288. doi: 10.1038/s41420-024-02048-6. Cell Death Discov. 2024. PMID: 38879630 Free PMC article.
-
Organoid co-culture model of the human endometrium in a fully synthetic extracellular matrix enables the study of epithelial-stromal crosstalk.Med. 2023 Aug 11;4(8):554-579.e9. doi: 10.1016/j.medj.2023.07.004. Med. 2023. PMID: 37572651 Free PMC article.
-
Loss of CDYL Results in Suppression of CTNNB1 and Decreased Endometrial Receptivity.Front Cell Dev Biol. 2020 Feb 25;8:105. doi: 10.3389/fcell.2020.00105. eCollection 2020. Front Cell Dev Biol. 2020. PMID: 32158757 Free PMC article.
-
Cabergoline Stimulates Human Endometrial Stromal Cell Decidualization and Reverses Effects of Interleukin-1β In Vitro.J Clin Endocrinol Metab. 2021 Nov 19;106(12):3591-3604. doi: 10.1210/clinem/dgab511. J Clin Endocrinol Metab. 2021. PMID: 34260712 Free PMC article.
References
-
- Bellofiore N, Ellery SJ, Mamrot J, Walker DW, Temple-Smith P, Dickinson H. First evidence of a menstruating rodent: the spiny mouse (Acomys cahirinus). Am J Obstet Gynecol. 2017;216(1):40.e1–40.e11. - PubMed
-
- Smith SK. Prostaglandins and growth factors in the endometrium. Baillieres Clin Obstet Gynaecol. 1989;3(2):249–270. - PubMed
-
- Shifren JL, Tseng JF, Zaloudek CJ, Ryan IP, Meng YG, Ferrara N, Jaffe RB, Taylor RN. Ovarian steroid regulation of vascular endothelial growth factor in the human endometrium: implications for angiogenesis during the menstrual cycle and in the pathogenesis of endometriosis. J Clin Endocrinol Metab. 1996;81(8):3112–3118. - PubMed
-
- Brosens JJ, Wilson MS, Lam EW. FOXO transcription factors: from cell fate decisions to regulation of human female reproduction. Adv Exp Med Biol. 2009;665:227–241. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

