Prenatal Diagnosis of Resistance to Thyroid Hormone and Its Clinical Implications
- PMID: 28938413
- PMCID: PMC5630247
- DOI: 10.1210/jc.2017-01251
Prenatal Diagnosis of Resistance to Thyroid Hormone and Its Clinical Implications
Abstract
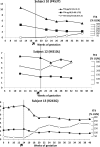
Context: Resistance to thyroid hormone-β (RTH-β) is an autosomal dominant disorder characterized by reduced sensitivity of target tissues to thyroid hormones (THs). Individuals with RTH-β have high TH levels usually due to mutations in the TH receptor-β (THRB) gene. The management of RTH-β during pregnancy is challenging, as wild-type (WT) fetuses born to RTH-β mothers have low birth weight and suppressed postnatal thyroid-stimulating hormone (TSH), due to intrauterine exposure to excess TH.
Objective: To determine birth weight and postnatal TSH of WT fetuses carried by mothers with RTH-β whose fT4 levels were maintained below 20% of the upper limit of normal (ULN).
Design: Retrospective chart review.
Setting: Academic institution in collaboration with off-site hospitals and private practices.
Patients: Thirteen women harboring THRB gene mutations were evaluated during 18 pregnancies.
Intervention: Prenatal genetic diagnosis by amniocentesis. Women carrying WT fetuses were given the option of treatment with antithyroid medication by their treating physicians with the aim to avoid serum fT4 levels above 20% of the ULN.
Results: No significant difference was found in birth weight corrected for gestational age and in serum TSH levels at birth between WT and RTH-β infants born to RTH-β mothers.
Conclusions: Prenatal diagnosis may play an important role in the management of RTH-β during pregnancy. Aiming for maternal fT4 levels not above 50% of the ULN in RTH-β mothers carrying WT fetuses seems to be a prudent approach that prevents the otherwise expected low birth weight and postnatal TSH suppression.
Copyright © 2017 Endocrine Society
Figures

Similar articles
-
Thyroid hormone resistance from newborns to adults: a Spanish experience.J Endocrinol Invest. 2019 Aug;42(8):941-949. doi: 10.1007/s40618-019-1007-4. Epub 2019 Feb 1. J Endocrinol Invest. 2019. PMID: 30707410
-
Fetal loss associated with excess thyroid hormone exposure.JAMA. 2004 Aug 11;292(6):691-5. doi: 10.1001/jama.292.6.691. JAMA. 2004. PMID: 15304465
-
Fetal Exposure to High Maternal Thyroid Hormone Levels Causes Central Resistance to Thyroid Hormone in Adult Humans and Mice.J Clin Endocrinol Metab. 2017 Sep 1;102(9):3234-3240. doi: 10.1210/jc.2017-00019. J Clin Endocrinol Metab. 2017. PMID: 28586435 Free PMC article.
-
Thyroid hormone resistance.Ann Clin Biochem. 2006 Nov;43(Pt 6):431-40. doi: 10.1258/000456306778904678. Ann Clin Biochem. 2006. PMID: 17132274 Review.
-
Update on resistance to thyroid hormone syndromeβ.Ital J Pediatr. 2020 Nov 11;46(1):168. doi: 10.1186/s13052-020-00929-x. Ital J Pediatr. 2020. PMID: 33176840 Free PMC article. Review.
Cited by
-
Thyroid hormone resistance from newborns to adults: a Spanish experience.J Endocrinol Invest. 2019 Aug;42(8):941-949. doi: 10.1007/s40618-019-1007-4. Epub 2019 Feb 1. J Endocrinol Invest. 2019. PMID: 30707410
-
Maternal Resistance to Thyroid Hormone β and Pregnancy Outcomes.J Clin Endocrinol Metab. 2023 Dec 21;109(1):e420-e421. doi: 10.1210/clinem/dgad350. J Clin Endocrinol Metab. 2023. PMID: 37315195 Free PMC article. No abstract available.
-
Epigenetic developmental programming and intergenerational effects of thyroid hormones.Vitam Horm. 2023;122:23-49. doi: 10.1016/bs.vh.2023.01.003. Epub 2023 Feb 9. Vitam Horm. 2023. PMID: 36863795 Free PMC article. Review.
-
Physiologic Significance of Epigenetic Regulation of Thyroid Hormone Target Gene Expression.Eur Thyroid J. 2020 May;9(3):114-123. doi: 10.1159/000506423. Epub 2020 Mar 24. Eur Thyroid J. 2020. PMID: 32523888 Free PMC article. Review.
-
Resistance to Thyroid Hormone Beta: A Focused Review.Front Endocrinol (Lausanne). 2021 Mar 31;12:656551. doi: 10.3389/fendo.2021.656551. eCollection 2021. Front Endocrinol (Lausanne). 2021. PMID: 33868182 Free PMC article. Review.
References
-
- Haddow JE, Palomaki GE, Allan WC, Williams JR, Knight GJ, Gagnon J, O’Heir CE, Mitchell ML, Hermos RJ, Waisbren SE, Faix JD, Klein RZ. Maternal thyroid deficiency during pregnancy and subsequent neuropsychological development of the child. N Engl J Med. 1999;341(8):549–555. - PubMed
-
- Pop VJ, Kuijpens JL, van Baar AL, Verkerk G, van Son MM, de Vijlder JJ, Vulsma T, Wiersinga WM, Drexhage HA, Vader HL. Low maternal free thyroxine concentrations during early pregnancy are associated with impaired psychomotor development in infancy. Clin Endocrinol (Oxf). 1999;50(2):149–155. - PubMed
-
- Phoojaroenchanachai M, Sriussadaporn S, Peerapatdit T, Vannasaeng S, Nitiyanant W, Boonnamsiri V, Vichayanrat A. Effect of maternal hyperthyroidism during late pregnancy on the risk of neonatal low birth weight. Clin Endocrinol (Oxf). 2001;54(3):365–370. - PubMed
-
- Dumitrescu AM, Refetoff S. Impaired sensitivity to thyroid hormone: defects of transport, metabolism and action. In: De Groot LJ, Beck-Peccoz P, Chrousos G, Dungan K, Grossman A, Hershman JM, Koch C, Korbonits M, McLachlan R, New M, Purnell J, Rebar R, Singer F, Vinik A, eds. Endotext. South Dartmouth, MA: MDText.com, Inc; 2000. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

