Sex- and Tissue-Specific Role of Estrogen Sulfotransferase in Energy Homeostasis and Insulin Sensitivity
- PMID: 28938414
- PMCID: PMC5695832
- DOI: 10.1210/en.2017-00571
Sex- and Tissue-Specific Role of Estrogen Sulfotransferase in Energy Homeostasis and Insulin Sensitivity
Abstract
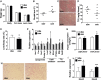
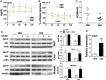
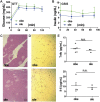
Estrogen sulfotransferase catalyzes the sulfoconjugation and deactivation of estrogens. Previously, we showed that loss of Est in male ob/ob mice, but not in female ob/ob mice, exacerbated the diabetic phenotype, but the underlying mechanism was unclear. In this study, we show that transgenic reconstitution of Est in the adipose tissue, but not in the liver, attenuated diabetic phenotype in Est-deficient ob/ob mice (obe mice). Mechanistically, adipose reconstitution of Est in obe mice (oae mice) resulted in reduced local and systemic inflammation, improved insulin sensitivity, and increased energy expenditure. At the molecular level, adipose induction of lipocalin-2 (Lcn2) in oae males may have contributed to the inhibition of inflammation because the level of Lcn2 was negatively associated with tumor necrosis factor (Tnf) α expression, and treatment of differentiated adipocytes with Lcn2 antagonized Tnfα-responsive inhibition of insulin signaling. The metabolic benefit of adipose reconstitution of Est was sex specific, because adipose reconstitution of Est in obe females had little effect. Interestingly, despite their improved metabolic functions, obe male mice with reconstituted Est in their adipose tissue failed to ameliorate the impairment of the structure and function of the pancreatic islets. In summary, our study uncovers a crucial adipose- and male-specific role of Est in maintaining the whole-body energy homeostasis.
Copyright © 2017 Endocrine Society.
Figures




Comment in
-
New Insights Into Estrogens Inactivation and Prevention of Systemic Inflammation in Male Subjects.Endocrinology. 2017 Nov 1;158(11):3711-3712. doi: 10.1210/en.2017-00828. Endocrinology. 2017. PMID: 29099958 Free PMC article. No abstract available.
Similar articles
-
Sex-specific effect of estrogen sulfotransferase on mouse models of type 2 diabetes.Diabetes. 2012 Jun;61(6):1543-51. doi: 10.2337/db11-1152. Epub 2012 Mar 20. Diabetes. 2012. PMID: 22438574 Free PMC article.
-
Estrogen sulfotransferase regulates body fat and glucose homeostasis in female mice.Am J Physiol Endocrinol Metab. 2010 Oct;299(4):E657-64. doi: 10.1152/ajpendo.00707.2009. Epub 2010 Aug 3. Am J Physiol Endocrinol Metab. 2010. PMID: 20682840 Free PMC article.
-
Gender-specific expression and mechanism of regulation of estrogen sulfotransferase in adipose tissues of the mouse.Endocrinology. 2008 Nov;149(11):5440-8. doi: 10.1210/en.2008-0271. Epub 2008 Jul 31. Endocrinology. 2008. PMID: 18669602 Free PMC article.
-
Sex-specific metabolic functions of adipose Lipocalin-2.Mol Metab. 2019 Dec;30:30-47. doi: 10.1016/j.molmet.2019.09.009. Epub 2019 Sep 27. Mol Metab. 2019. PMID: 31767179 Free PMC article.
-
Sex-Dependent Role of Estrogen Sulfotransferase and Steroid Sulfatase in Metabolic Homeostasis.Adv Exp Med Biol. 2017;1043:455-469. doi: 10.1007/978-3-319-70178-3_21. Adv Exp Med Biol. 2017. PMID: 29224107 Review.
Cited by
-
Lipocalin-2 counteracts metabolic dysregulation in obesity and diabetes.J Exp Med. 2020 Oct 5;217(10):e20191261. doi: 10.1084/jem.20191261. J Exp Med. 2020. PMID: 32639539 Free PMC article.
-
Hepatic Estrogen Sulfotransferase Distantly Sensitizes Mice to Hemorrhagic Shock-Induced Acute Lung Injury.Endocrinology. 2020 Jan 1;161(1):bqz031. doi: 10.1210/endocr/bqz031. Endocrinology. 2020. PMID: 31837219 Free PMC article.
-
Estrogen sulfotransferase in the metabolism of estrogenic drugs and in the pathogenesis of diseases.Expert Opin Drug Metab Toxicol. 2019 Apr;15(4):329-339. doi: 10.1080/17425255.2019.1588884. Epub 2019 Mar 18. Expert Opin Drug Metab Toxicol. 2019. PMID: 30822161 Free PMC article.
-
Do Lifestyle Interventions Mitigate the Oxidative Damage and Inflammation Induced by Obesity in the Testis?Antioxidants (Basel). 2025 Jan 27;14(2):150. doi: 10.3390/antiox14020150. Antioxidants (Basel). 2025. PMID: 40002337 Free PMC article. Review.
-
Sex-Dimorphic and Sex Hormone-Dependent Role of Steroid Sulfatase in Adipose Inflammation and Energy Homeostasis.Endocrinology. 2018 Sep 1;159(9):3365-3377. doi: 10.1210/en.2018-00531. Endocrinology. 2018. PMID: 30060148 Free PMC article.
References
-
- Blüher M. Adipose tissue inflammation: a cause or consequence of obesity-related insulin resistance? Clin Sci (Lond). 2016;130(18):1603–1614. - PubMed
-
- Sargent J. Obesity: rethinking inflammation and adipocyte homeostasis. Nat Rev Endocrinol. 2014;10(8):446. - PubMed
-
- Kahn SE, Hull RL, Utzschneider KM. Mechanisms linking obesity to insulin resistance and type 2 diabetes. Nature. 2006;444(7121):840–846. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

