CD3xPDL1 bi-specific T cell engager (BiTE) simultaneously activates T cells and NKT cells, kills PDL1+ tumor cells, and extends the survival of tumor-bearing humanized mice
- PMID: 28938530
- PMCID: PMC5601626
- DOI: 10.18632/oncotarget.19865
CD3xPDL1 bi-specific T cell engager (BiTE) simultaneously activates T cells and NKT cells, kills PDL1+ tumor cells, and extends the survival of tumor-bearing humanized mice
Abstract
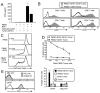
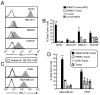
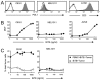
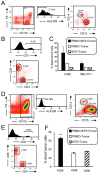
Bi-specific T cell engagers (BiTEs) activate T cells through CD3 and target activated T cells to tumor-expressed antigens. BiTEs have shown therapeutic efficacy in patients with liquid tumors; however, they do not benefit all patients. Anti-tumor immunity is limited by Programmed Death 1 (PD1) pathway-mediated immune suppression, and patients who do not benefit from existing BiTES may be non-responders because their T cells are anergized via the PD1 pathway. We have designed a BiTE that activates and targets both T cells and NKT cells to PDL1+ cells. In vitro studies demonstrate that the CD3xPDL1 BiTE simultaneously binds to both CD3 and PDL1, and activates healthy donor CD4+ and CD8+ T cells and NKT cells that are specifically cytotoxic for PDL1+ tumor cells. Cancer patients' PBMC are also activated and cytotoxic, despite the presence of myeloid-derived suppressor cells. The CD3xPDL1 BiTE significantly extends the survival time and maintains activated immune cell levels in humanized NSG mice reconstituted with human PBMC and carrying established human melanoma tumors. These studies suggest that the CD3xPDL1 BiTE may be efficacious for patients with PDL1+ solid tumors, in combination with other immunotherapies that do not specifically neutralize PD1 pathway-mediated immune suppression.
Keywords: T cell activation; cancer immunotherapy; checkpoint inhibitor blockade; solid tumors; tumor-induced immune suppression.
Conflict of interest statement
Conflicts of Interest The authors have no conflicts of interest.
Figures



References
-
- Baeuerle PA, Reinhardt C. Bispecific T-cell engaging antibodies for cancer therapy. Cancer Res. 2009;69:4941–4944. - PubMed
-
- Loffler A, Kufer P, Lutterbuse R, Zettl F, Daniel PT, Schwenkenbecher JM, Riethmuller G, Dorken B, Bargou RC. A recombinant bispecific single-chain antibody, CD19 x CD3, induces rapid and high lymphoma-directed cytotoxicity by unstimulated T lymphocytes. Blood. 2000;95:2098–2103. - PubMed
-
- Schlereth B, Kleindienst P, Fichtner I, Lorenczewski G, Brischwein K, Lippold S, da Silva A, Locher M, Kischel R, Lutterbuse R, Kufer P, Baeuerle PA. Potent inhibition of local and disseminated tumor growth in immunocompetent mouse models by a bispecific antibody construct specific for Murine CD3. Cancer Immunol Immunother. 2006;55:785–796. - PMC - PubMed
-
- Dreier T, Baeuerle PA, Fichtner I, Grun M, Schlereth B, Lorenczewski G, Kufer P, Lutterbuse R, Riethmuller G, Gjorstrup P, Bargou RC. T cell costimulus-independent and very efficacious inhibition of tumor growth in mice bearing subcutaneous or leukemic human B cell lymphoma xenografts by a CD19-/CD3- bispecific single-chain antibody construct. J Immunol. 2003;170:4397–4402. - PubMed
-
- Dreier T, Lorenczewski G, Brandl C, Hoffmann P, Syring U, Hanakam F, Kufer P, Riethmuller G, Bargou R, Baeuerle PA. Extremely potent, rapid and costimulation-independent cytotoxic T-cell response against lymphoma cells catalyzed by a single-chain bispecific antibody. Int J Cancer. 2002;100:690–697. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

