Facile total synthesis of lysicamine and the anticancer activities of the RuII, RhIII, MnII and ZnII complexes of lysicamine
- PMID: 28938642
- PMCID: PMC5601738
- DOI: 10.18632/oncotarget.19584
Facile total synthesis of lysicamine and the anticancer activities of the RuII, RhIII, MnII and ZnII complexes of lysicamine
Abstract
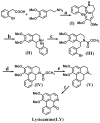
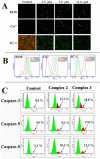
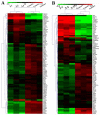
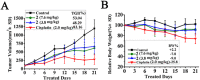
Lysicamine is a natural oxoaporphine alkaloid, which isolated from traditional Chinese medicine (TCM) herbs and has been shown to possess cytotoxicity to hepatocarcinoma cell lines. Reports on its antitumor activity are scarce because lysicamine occurs in plants at a low content. In this work, we demonstrate a facile concise total synthesis of lysicamine from simple raw materials under mild reaction conditions, and the preparation of the Ru(II), Rh(III), Mn(II) and Zn(II) complexes 1-4 of lysicamine (LY). All the compounds were fully characterized by elemental analysis, IR, ESI-MS, 1H and 13C NMR, as well as single-crystal X-ray diffraction analysis. Compared with the free ligand LY, complexes 2 and 3 exhibited superior in vitro cytotoxicity against HepG2 and NCI-H460. Mechanistic studies indicated that 2 and 3 blocked the cell cycle in the S phase by decreasing of cyclins A2/B1/D1/E1, CDK 2/6, and PCNA levels and increasing levels of p21, p27, p53 and CDC25A proteins. In addition, 2 and 3 induced cell apoptosis via both the caspase-dependent mitochondrial pathway and the death receptor pathway. in vivo study showed that 2 inhibited HepG2 tumor growth at 1/3 maximum tolerated dose (MTD) and had a better safety profile than cisplatin.
Keywords: antitumor activity; apoptosis; lysicamine; metal complexes.
Conflict of interest statement
CONFLICTS OF INTEREST The authors declared that they have no conflicts of interest to this work.
Figures




Similar articles
-
Water-soluble oxoglaucine-Y(III), Dy(III) complexes: in vitro and in vivo anticancer activities by triggering DNA damage, leading to S phase arrest and apoptosis.Dalton Trans. 2015 Jul 7;44(25):11408-19. doi: 10.1039/c5dt00926j. Dalton Trans. 2015. PMID: 26017376
-
Divalent later transition metal complexes of the traditional chinese medicine (TCM) liriodenine: coordination chemistry, cytotoxicity and DNA binding studies.Dalton Trans. 2009 Dec 28;(48):10813-23. doi: 10.1039/b912553a. Epub 2009 Nov 6. Dalton Trans. 2009. PMID: 20023911
-
Synthesis, characterization and anticancer effect of the ruthenium (II) polypyridyl complexes on HepG2 cells.J Photochem Photobiol B. 2016 Dec;165:246-255. doi: 10.1016/j.jphotobiol.2016.10.038. Epub 2016 Oct 31. J Photochem Photobiol B. 2016. PMID: 27816647
-
Effect of glucosamine conjugation to zinc(II) complexes of a bis-pyrazole ligand: syntheses, characterization and anticancer activity.J Inorg Biochem. 2014 Nov;140:131-42. doi: 10.1016/j.jinorgbio.2014.07.009. Epub 2014 Jul 22. J Inorg Biochem. 2014. PMID: 25113858
-
Thiosemicarbazone Cu(II) and Zn(II) complexes as potential anticancer agents: syntheses, crystal structure, DNA cleavage, cytotoxicity and apoptosis induction activity.J Inorg Biochem. 2014 Jul;136:13-23. doi: 10.1016/j.jinorgbio.2014.03.004. Epub 2014 Mar 16. J Inorg Biochem. 2014. PMID: 24690556
Cited by
-
Ruthenium Complexes as Promising Candidates against Lung Cancer.Molecules. 2021 Jul 21;26(15):4389. doi: 10.3390/molecules26154389. Molecules. 2021. PMID: 34361543 Free PMC article. Review.
-
Lysicamine Reduces Protein Kinase B (AKT) Activation and Promotes Necrosis in Anaplastic Thyroid Cancer.Pharmaceuticals (Basel). 2023 Dec 4;16(12):1687. doi: 10.3390/ph16121687. Pharmaceuticals (Basel). 2023. PMID: 38139812 Free PMC article.
References
-
- Jantan I, Raweh SM, Yasin YH, Murad S. Antiplatelet activity of aporphine and phenanthrenoid alkaloids from Aromadendron elegans Blume. Phytother Res. 2006;20:493–496. - PubMed
-
- Remichkova M, Dimitrova P, Philipov S, Ivanovska N. Toll-like receptor-mediated anti-inflammatory action of glaucine and oxoglaucine. Fitoterapia. 2009;80:411–414. - PubMed
-
- Wirasathien L, Boonarkart C, Pengsuparp T, Suttisri R. Biological activities of alkaloids from Pseuduvaria setosa. Pharm Biol. 2006;44:274–278.
-
- Yang CH, Cheng MJ, Lee SJ, Yang CW, Chang HS, Chen IS. Secondary metabolites and cytotoxic activities from the stem bark of Zanthoxylum nitidum. Chem Biodivers. 2009;6:846–857. - PubMed
-
- Hsieh TJ, Liu TZ, Chern CL, Tsao DA, Lu FJ, Syu YH, Hsieh PY, Hu HS, Chang TT, Chen CH. Liriodenine inhibits the proliferation of human hepatoma cell lines by blocking cell cycle progression and nitric oxide-mediated activation of p53 expression. Food Chem Toxicol. 2005;43:1117–1126. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

