Efficacy and safety of traditional chemotherapies for patients with ovarian neoplasm: a network meta-analysis
- PMID: 28938689
- PMCID: PMC5601785
- DOI: 10.18632/oncotarget.16729
Efficacy and safety of traditional chemotherapies for patients with ovarian neoplasm: a network meta-analysis
Abstract
Background: Ovarian neoplasm is a kind of high risky cancer among female. This paper assessed the efficacy and safety of twelve therapies and figured out the superior chemotherapeutic drug for ovarian cancer through network meta-analysis (NMA).
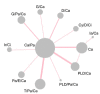
Method: Eligible randomized controlled trials (RCTs) were retrieved from electronic databases. Primary outcomes concerning efficacy, overall survival (OS) and progression-free survival (PFS), were presented as hazard ratio (HR) and the associated 95% credible interval(CrI), while outcomes concerning safety were assessed by odds ratio (OR) and the corresponding 95% CrI. Surface under the cumulative ranking curve (SUCRA) was calculated under each survival and safety outcome in order to show the rankings of tested therapies.
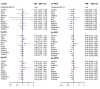
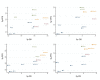
Result: Electronic databases such as PubMed and Embase were searched to finally obtain 19 eligible studies of 16290 patients. In accordance of primary outcomes, when it came to 3-y PFS, paclitaxel/epirubicin/carboplatin (Pa/E/Ca) and pegylated liposomal doxorubicin/ paclitaxel/ carboplatin (PLD/Pa/Ca) were preferred compared to carboplatin (Ca) (HR= 0.80, 95% CrI= 0.67-0.96; HR= 0.83, 95% CrI= 0.69-0.99). According to 5y-PFS, Pa/E/Ca was notably better than Ca (HR= 0.80, 95% CrI= 0.65-0.99). As to adverse effects, Ca was superior to Pa/E/Ca in neuropathy (HR=0.05, 95% CrI=0.02-0.19). Pa/E/Ca showed high rankings in 3y-PFS (SUCRA=0.749), 5y-OS (SUCRA=0.738) and 5y-PFS (SUCRA=0.798) while (PLD/Pa/Ca) in 3y-OS (SUCRA=0.737), 5y-OS (SUCRA=0.687) and 5y-PFS (SUCRA=0.712). Besides, Pa/E/Ca ranked the third with a SUCRA of 0.661 in neutropenia.
Conclusion: PLD/Pa/Ca, PLD/Ca and Pa/E/Ca are highly recommended as potential therapeutically choices for patients with ovarian cancer. But considering the lack of safety data for PLD/Pa/Ca, this intervention should be taken with caution.
Keywords: carboplatin; network meta-analysis; ovarian neoplasm; paclitaxel; pegylated liposomal doxorubicin.
Conflict of interest statement
CONFLICTS OF INTEREST The authors declare no financial interests in the findings described in the manuscript.
Figures
Similar articles
-
Carboplatin plus paclitaxel versus carboplatin plus pegylated liposomal doxorubicin as first-line treatment for patients with ovarian cancer: the MITO-2 randomized phase III trial.J Clin Oncol. 2011 Sep 20;29(27):3628-35. doi: 10.1200/JCO.2010.33.8566. Epub 2011 Aug 15. J Clin Oncol. 2011. PMID: 21844495 Clinical Trial.
-
Comparative Effectiveness of Neoadjuvant Treatments for Resectable Gastroesophageal Cancer: A Network Meta-Analysis.Front Pharmacol. 2018 Aug 6;9:872. doi: 10.3389/fphar.2018.00872. eCollection 2018. Front Pharmacol. 2018. PMID: 30127746 Free PMC article.
-
Combinational use of trabectedin and pegylated liposomal doxorubicin for recurrent ovarian cancer: a meta-analysis of phase III randomized controlled trials.Am J Transl Res. 2023 Dec 15;15(12):6675-6689. eCollection 2023. Am J Transl Res. 2023. PMID: 38186978 Free PMC article. Review.
-
Carboplatin and pegylated liposomal doxorubicin versus carboplatin and paclitaxel in very platinum-sensitive ovarian cancer patients: results from a subset analysis of the CALYPSO phase III trial.Eur J Cancer. 2015 Feb;51(3):352-8. doi: 10.1016/j.ejca.2014.11.017. Epub 2014 Dec 17. Eur J Cancer. 2015. PMID: 25534295 Clinical Trial.
-
Restricted Versus Liberal Versus Goal-Directed Fluid Therapy for Non-vascular Abdominal Surgery: A Network Meta-Analysis and Systematic Review.Cureus. 2023 Apr 28;15(4):e38238. doi: 10.7759/cureus.38238. eCollection 2023 Apr. Cureus. 2023. PMID: 37261162 Free PMC article. Review.
References
-
- Salehi F, Dunfield L, Phillips KP, Krewski D, Vanderhyden BC. Risk factors for ovarian cancer: an overview with emphasis on hormonal factors. Journal of toxicology and environmental health Part B, Critical reviews. 2008;11:301–321. - PubMed
-
- Bhatt P, Vhora I, Patil S, Amrutiya J, Bhattacharya C, Misra A, Mashru R. Role of antibodies in diagnosis and treatment of ovarian cancer: Basic approach and clinical status. Journal of controlled release. 2016;226:148–167. - PubMed
-
- Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A. Global cancer statistics, 2012. CA: a cancer journal for clinicians. 2015;65:87–108. - PubMed
-
- Hunn J, Rodriguez GC. Ovarian cancer: etiology, risk factors, and epidemiology. Clinical obstetrics and gynecology. 2012;55:3–23. - PubMed
-
- Parmar MK, Ledermann JA, Colombo N, du Bois A, Delaloye JF, Kristensen GB, Wheeler S, Swart AM, Qian W, Torri V, Floriani I, Jayson G, Lamont A, et al. Lancet. Vol. 361. London, England: 2003. Paclitaxel plus platinum-based chemotherapy versus conventional platinum-based chemotherapy in women with relapsed ovarian cancer: the ICON4/AGO-OVAR-2.2 trial; pp. 2099–2106. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

