Effectiveness and safety of PD-1/PD-L1 inhibitors in the treatment of solid tumors: a systematic review and meta-analysis
- PMID: 28938692
- PMCID: PMC5601788
- DOI: 10.18632/oncotarget.18316
Effectiveness and safety of PD-1/PD-L1 inhibitors in the treatment of solid tumors: a systematic review and meta-analysis
Abstract
Background: PD-1/PD-L1 inhibitors have been implicated as potentially effective anti-cancer therapies. Some clinical randomized controlled trials (RCTs) have been completed for a variety of PD-1/PD-L1 inhibitors to treat various malignancies, and more RCTs are still under way. We carried out this systematic meta-analysis to evaluate the efficacy and safety of PD-1/PD-L1 inhibitors in the treatment of solid tumors.
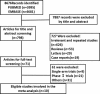
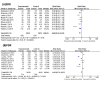
Methods: We searched PubMed, EMBASE, clinical trial registers, conference reports, and related reviews. Eligible RCTs that compared PD-1/PD-L1 inhibitors with other chemotherapy agents or placebo in solid tumor patients were included. For each RCT, progression-free survival (PFS), overall survival (OS), objective response rate (ORR), disease control rate (DCR), stable disease rate (SDR), progressive disease rate (PDR), and adverse events (AEs) were pooled for meta-analysis.
Findings: Based on an analysis of 10 eligible RCTs, PD-1/PD-L1 inhibitors were found to significantly improve PFS (Hazard ratio (HR), 0.65; 95% confidence interval (CI) 0.53 to 0.79, P<0.001), OS (HR, 0.69; 95%CI 0.62 to 0.76, P<0.001), and ORR (Risk Ratio (RR) 292; 95% confidence interval (CI) 2.06 to 4.15, P<0.00001) in all populations, including melanoma and NSCLC subgroups. However, they failed to increase the DCR of cancer patients (RR 1.15; 95%CI 0.91 to 1.45, P=0.25). Furthermore, less AEs were observed in the PD-1/PD-L1 inhibitor groups than the control groups.
Interpretation: PD-1 inhibitors are more effective for improving the PFS, OS, and ORR of cancer patients with little toxicity, despite having little effect on increasing of the DCR.
Keywords: PD-1; PD-L1; cancer; nivolumab; pembrolizumab.
Conflict of interest statement
CONFLICTS OF INTEREST The authors declare no conflicts of interest in this work.
Figures






Similar articles
-
Effectiveness and safety of PD-1/PD-L1 or CTLA4 inhibitors combined with chemotherapy as a first-line treatment for lung cancer: A meta-analysis.J Thorac Dis. 2018 Dec;10(12):6636-6652. doi: 10.21037/jtd.2018.11.72. J Thorac Dis. 2018. PMID: 30746209 Free PMC article.
-
Efficacy and safety of anti-PD-1/anti-PD-L1 antibody therapy in treatment of advanced gastric cancer or gastroesophageal junction cancer: A meta-analysis.World J Gastrointest Oncol. 2020 Nov 15;12(11):1346-1363. doi: 10.4251/wjgo.v12.i11.1346. World J Gastrointest Oncol. 2020. PMID: 33250966 Free PMC article.
-
Anti-PD-1/PD-L1 antibody versus conventional chemotherapy for previously-treated, advanced non-small-cell lung cancer: a meta-analysis of randomized controlled trials.J Thorac Dis. 2017 Mar;9(3):655-665. doi: 10.21037/jtd.2017.03.104. J Thorac Dis. 2017. PMID: 28449473 Free PMC article.
-
The Relative Risk of Immune-Related Liver Dysfunction of PD-1/PD-L1 Inhibitors Versus Chemotherapy in Solid Tumors: A Meta-Analysis of Randomized Controlled Trials.Front Pharmacol. 2019 Sep 23;10:1063. doi: 10.3389/fphar.2019.01063. eCollection 2019. Front Pharmacol. 2019. PMID: 31607917 Free PMC article.
-
PD-1/PD-L1 inhibitors plus bevacizumab plus chemotherapy versus PD-1/PD-L1 inhibitors plus chemotherapy for advanced non-small cell lung cancer: a phase 3 RCT based meta-analysis.Front Oncol. 2025 May 21;15:1496611. doi: 10.3389/fonc.2025.1496611. eCollection 2025. Front Oncol. 2025. PMID: 40469183 Free PMC article.
Cited by
-
Exceptional response to nivolumab of a heavily pre-treated patient with metastatic renal-cell cancer: from a case report to molecular investigation and future perspectives.Ther Adv Med Oncol. 2020 Aug 11;12:1758835920946152. doi: 10.1177/1758835920946152. eCollection 2020. Ther Adv Med Oncol. 2020. PMID: 32849917 Free PMC article.
-
Nivolumab-induced cold agglutinin syndrome successfully treated with rituximab.Blood Adv. 2018 Aug 14;2(15):1865-1868. doi: 10.1182/bloodadvances.2018019000. Blood Adv. 2018. PMID: 30072374 Free PMC article.
-
The introduction of LAG-3 checkpoint blockade in melanoma: immunotherapy landscape beyond PD-1 and CTLA-4 inhibition.Ther Adv Med Oncol. 2023 Jul 17;15:17588359231186027. doi: 10.1177/17588359231186027. eCollection 2023. Ther Adv Med Oncol. 2023. PMID: 37484526 Free PMC article. Review.
-
Activation of PGE2/EP2 and PGE2/EP4 signaling pathways positively regulate the level of PD-1 in infiltrating CD8+ T cells in patients with lung cancer.Oncol Lett. 2018 Jan;15(1):552-558. doi: 10.3892/ol.2017.7279. Epub 2017 Oct 26. Oncol Lett. 2018. PMID: 29285200 Free PMC article.
-
The Aryl hydrocarbon receptor mediates tobacco-induced PD-L1 expression and is associated with response to immunotherapy.Nat Commun. 2019 Mar 8;10(1):1125. doi: 10.1038/s41467-019-08887-7. Nat Commun. 2019. PMID: 30850589 Free PMC article.
References
-
- Dietrich K, Theobald M. [Immunological tumor therapy] Der Internist. 2015;56:907–916. quiz 917. - PubMed
-
- Zou W, Chen L. Inhibitory B7-family molecules in the tumour microenvironment. Nature reviews Immunology. 2008;8:467–477. - PubMed
-
- Yamazaki T, Akiba H, Iwai H, Matsuda H, Aoki M, Tanno Y, Shin T, Tsuchiya H, Pardoll DM, Okumura K, Azuma M, Yagita H. Expression of Programmed Death 1 Ligands by Murine T Cells and APC. The Journal of Immunology. 2002;169:5538–5545. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

