Hormone Therapy Use and Risk of Chronic Disease in the Nurses' Health Study: A Comparative Analysis With the Women's Health Initiative
- PMID: 28938710
- PMCID: PMC5860527
- DOI: 10.1093/aje/kwx131
Hormone Therapy Use and Risk of Chronic Disease in the Nurses' Health Study: A Comparative Analysis With the Women's Health Initiative
Erratum in
-
RE: "HORMONE THERAPY USE AND RISK OF CHRONIC DISEASE IN THE NURSES' HEALTH STUDY: A COMPARATIVE ANALYSIS WITH THE WOMEN'S HEALTH INITIATIVE".Am J Epidemiol. 2018 Mar 1;187(3):636. doi: 10.1093/aje/kwx364. Am J Epidemiol. 2018. PMID: 29506197 Free PMC article. No abstract available.
Abstract
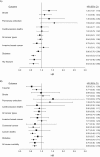
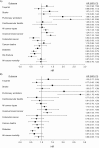
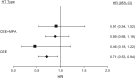
Observational studies and randomized controlled trials of menopausal hormone therapy (HT) and chronic disease risk appear to have divergent results for cardiovascular disease. However, differences may be related to a modifying effect of age, time since menopause, and HT formulation. In the Nurses' Health Study (NHS) (enrolling during 1980-1994 and following participants until 2002), we investigated associations between the use of oral conjugated equine estrogens (CEE) (0.625 mg/day) plus medroxyprogesterone acetate (MPA) (<10 mg/day) or oral CEE alone and cardiovascular disease, cancer, all-cause mortality, and other major endpoints among postmenopausal women, aged 50-79 years at HT initiation. Among women aged 50-59 years at HT initiation, associations of CEE alone or CEE+MPA with most clinical outcomes were highly concordant between NHS and Women's Health Initiative (WHI). However, for myocardial infarction, results for CEE+MPA were in the direction of risk elevation in WHI and in the direction of risk reduction in NHS. When examined according to years since menopause onset (<10 years) rather than age group, results were nonsignificant and concordant for both studies. Because few women in the NHS initiated HT after age 60 years, we did not examine associations in this group. Discrepancies between NHS and WHI could largely be attributed to differences in the age structure of the populations and age at HT initiation.
Keywords: Nurses’ Health Study; Women's Health Initiative; cardiovascular disease; chronic disease; epidemiologic methods; hormone therapy; randomized controlled trials.
© The Author(s) 2017. Published by Oxford University Press on behalf of the Johns Hopkins Bloomberg School of Public Health. All rights reserved. For permissions, please e-mail: journals.permissions@oup.com.
Figures
Similar articles
-
The effect of medroxyprogesterone acetate on estrogen-dependent risks and benefits--an attempt to interpret the Women's Health Initiative results.Gynecol Endocrinol. 2006 Jun;22(6):303-17. doi: 10.1080/09513590600717368. Gynecol Endocrinol. 2006. PMID: 16785155
-
Menopausal hormone therapy and health outcomes during the intervention and extended poststopping phases of the Women's Health Initiative randomized trials.JAMA. 2013 Oct 2;310(13):1353-68. doi: 10.1001/jama.2013.278040. JAMA. 2013. PMID: 24084921 Free PMC article. Clinical Trial.
-
Metabolomic Effects of Hormone Therapy and Associations With Coronary Heart Disease Among Postmenopausal Women.Circ Genom Precis Med. 2020 Dec;13(6):e002977. doi: 10.1161/CIRCGEN.119.002977. Epub 2020 Nov 3. Circ Genom Precis Med. 2020. PMID: 33141616 Free PMC article.
-
The Women's Health Initiative trial and related studies: 10 years later: a clinician's view.J Steroid Biochem Mol Biol. 2014 Jul;142:4-11. doi: 10.1016/j.jsbmb.2013.10.009. Epub 2013 Oct 27. J Steroid Biochem Mol Biol. 2014. PMID: 24172877 Review.
-
The Women's Health Initiative Randomized Trials and Clinical Practice: A Review.JAMA. 2024 May 28;331(20):1748-1760. doi: 10.1001/jama.2024.6542. JAMA. 2024. PMID: 38691368 Review.
Cited by
-
Causal inference in survival analysis using longitudinal observational data: Sequential trials and marginal structural models.Stat Med. 2023 Jun 15;42(13):2191-2225. doi: 10.1002/sim.9718. Epub 2023 Apr 22. Stat Med. 2023. PMID: 37086186 Free PMC article.
-
Cardiovascular health and the menopausal woman: the role of estrogen and when to begin and end hormone treatment.F1000Res. 2019 Sep 3;8:F1000 Faculty Rev-1576. doi: 10.12688/f1000research.15548.1. eCollection 2019. F1000Res. 2019. PMID: 31543950 Free PMC article. Review.
-
The impact of patient characteristics and lifestyle factors on the risk of an ipsilateral event after a primary DCIS: A systematic review.Breast. 2020 Apr;50:95-103. doi: 10.1016/j.breast.2020.02.006. Epub 2020 Feb 19. Breast. 2020. PMID: 32120064 Free PMC article.
-
Estrogen receptor profiles across tissues from male and female Rattus norvegicus.Biol Sex Differ. 2019 Jan 11;10(1):4. doi: 10.1186/s13293-019-0219-9. Biol Sex Differ. 2019. PMID: 30635056 Free PMC article.
-
RE: "HORMONE THERAPY USE AND RISK OF CHRONIC DISEASE IN THE NURSES' HEALTH STUDY: A COMPARATIVE ANALYSIS WITH THE WOMEN'S HEALTH INITIATIVE".Am J Epidemiol. 2018 Mar 1;187(3):636. doi: 10.1093/aje/kwx364. Am J Epidemiol. 2018. PMID: 29506197 Free PMC article. No abstract available.
References
-
- Hunt K, Vessey M, McPherson K. Mortality in a cohort of long-term users of hormone replacement therapy: an updated analysis. Br J Obstet Gynaecol. 1990;97(12):1080–1086. - PubMed
-
- Colditz GA, Hankinson SE, Hunter DJ, et al. . The use of estrogens and progestins and the risk of breast cancer in postmenopausal women. N Engl J Med. 1995;332(24):1589–1593. - PubMed
-
- Wu O. Postmenopausal hormone replacement therapy and venous thromboembolism. Gend Med. 2005;2(suppl A):S18–S27. - PubMed
-
- Grodstein F, Stampfer MJ, Falkeborn M, et al. . Postmenopausal hormone therapy and risk of cardiovascular disease and hip fracture in a cohort of Swedish women. Epidemiology. 1999;10(5):476–480. - PubMed
-
- Grodstein F, Manson JE, Colditz GA, et al. . A prospective, observational study of postmenopausal hormone therapy and primary prevention of cardiovascular disease. Ann Intern Med. 2000;133(12):933–941. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

