Complex CatSper-dependent and independent [Ca2+]i signalling in human spermatozoa induced by follicular fluid
- PMID: 28938737
- PMCID: PMC5850303
- DOI: 10.1093/humrep/dex269
Complex CatSper-dependent and independent [Ca2+]i signalling in human spermatozoa induced by follicular fluid
Abstract
Study question: Does progesterone in human follicular fluid (hFF) activate CatSper and do other components of hFF modulate this effect and/or contribute separately to hFF-induced Ca2+ signaling?
Summary answer: hFF potently stimulates CatSper and increases [Ca2+]i, primarily due to high concentrations of progesterone, however, other components of hFF also contribute to [Ca2+]i signaling, including modulation of CatSper channel activity and inhibition of [Ca2+]i oscillations.
What is known already: CatSper, the principal Ca2+ channel in spermatozoa, is progesterone-sensitive and essential for fertility. Both hFF and progesterone, which is present in hFF, influence sperm function and increase their [Ca2+]i.
Study design, size, duration: This basic medical research study used semen samples from >40 donors and hFF from >50 patients who were undergoing surgical oocyte retrieval for IVF/ICSI.
Participants/materials, setting, methods: Semen donors and patients were recruited in accordance with local ethics approval (13/ES/0091) from the East of Scotland Research Ethics Service REC1. Activities of CatSper and KSper were assessed by patch clamp electrophysiology. Sperm [Ca2+]i responses were examined in sperm populations and single cells. Computer-assisted sperm analysis (CASA) parameters and penetration into viscous media were used to assess functional effects.
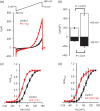
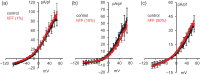
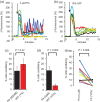
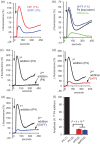
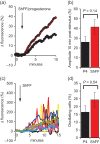
Main results and the role of chance: hFF and progesterone significantly potentiated CatSper currents. Under quasi-physiological conditions, hFF (up to 50%) failed to alter membrane K+ conductance or current reversal potential. hFF and progesterone (at an equivalent concentration) stimulated similar biphasic [Ca2+]i signals both in sperm populations and single cells. At a high hFF concentration (10%), the sustained (plateau) component of the [Ca2+]i signal was consistently greater than that induced by progesterone alone. In single cell recordings, 1% hFF-induced [Ca2+]i oscillations similarly to progesterone but with 10% hFF generation of [Ca2+]i oscillations was suppressed. After treatment to 'strip' lipid-derived mediators, hFF failed to significantly stimulate CatSper currents but induced small [Ca2+]i responses that were greater than those induced by the equivalent concentration of progesterone after stripping. Similar [Ca2+]i responses were observed when sperm pretreated with 3 μM progesterone (to desensitize progesterone responses) were stimulated with hFF or stripped hFF. hFF stimulated viscous media penetration and was more effective than the equivalent does of progesterone.
Large scale data: N/A.
Limitations, reasons for caution: This was an in vitro study. Caution must be taken when extrapolating these results in vivo.
Wider implications of the findings: This study directly demonstrates that hFF activates CatSper and establishes that the biologically important effects of hFF reflect, at least in part, action on this channel, primarily via progesterone. However, these experiments also demonstrate that other components of hFF both contribute to the [Ca2+]i signal and modulate the activation of CatSper. Simple in vitro experiments performed out of the context of the complex in vivo environment need to be interpreted with caution.
Study funding/competing interest(s): Funding was provided by MRC (MR/K013343/1, MR/012492/1) (S.G.B., S.J.P., C.L.R.B.) and University of Abertay (sabbatical for S.G.B.). Additional funding was provided by TENOVUS SCOTLAND (S.M.D.S.), Chief Scientist Office/NHS Research Scotland (S.M.D.S). C.L.R.B. is EIC of MHR and Chair of the WHO ESG on Diagnosis of Male infertility. The remaining authors have no conlicts of interest.
Keywords: CatSper; follicular fluid; patch clamp electrophysiology; potassium channel; spermatozoa.
© The Author 2017. Published by Oxford University Press on behalf of the European Society of Human Reproduction and Embryology.
Figures


References
-
- Aitken RJ, Buckingham DW, Irvine DS. The extragenomic action of progesterone on human spermatozoa: evidence for a ubiquitous response that is rapidly down-regulated. Endocrinology 1996;137:3999–4009. - PubMed
-
- Baldi E, Luconi M, Bonaccorsi L, Forti G. Nongenomic effects of progesterone on spermatozoa: mechanisms of signal transduction and clinical implications. Front Biosci 1998;3:D1051–D1059. - PubMed
-
- Bedu-Addo K, Barratt CL, Kirkman-Brown JC, Publicover SJ. Patterns of [Ca2+]i mobilization and cell response in human spermatozoa exposed to progesterone. Dev Biol 2007;302:324–332. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

