Treatment of lumbar degenerative disc disease-associated radicular pain with culture-expanded autologous mesenchymal stem cells: a pilot study on safety and efficacy
- PMID: 28938891
- PMCID: PMC5610473
- DOI: 10.1186/s12967-017-1300-y
Treatment of lumbar degenerative disc disease-associated radicular pain with culture-expanded autologous mesenchymal stem cells: a pilot study on safety and efficacy
Abstract
Background: Degenerative disc disease (DDD) is a common cause of lower back pain with radicular symptoms and has a significant socioeconomic impact given the associated disability. Limited effective conservative therapeutic options result in many turning to surgical alternatives for management, which vary in the rate of success and also carry an increased risk of morbidity and mortality associated with the procedures. Several animal based studies and a few human pilot studies have demonstrated safety and suggest efficacy in the treatment of DDD with mesenchymal stem cells (MSCs). The use of bone marrow-derived MSCs for the treatment of DDD is promising and in the present study we report on the safety and efficacy findings from a registry based proof of concept study using a percutaneous intradiscal injection of cultured MSCs for the management of DDD with associated radicular symptoms.
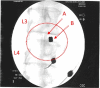
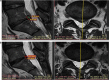
Methods: Thirty-three patients with lower back pain and disc degeneration with a posterior disc bulge diagnosed on magnetic resonance imaging (MRI) met the inclusion criteria and were treated with culture-expanded, autologous, bone marrow-derived MSCs. Prospective registry data was obtained at multiple time intervals up to 6 years post-treatment. Collected outcomes included numeric pain score (NPS), a modified single assessment numeric evaluation (SANE) rating, functional rating index (FRI), measurement of the intervertebral disc posterior dimension, and adverse events.
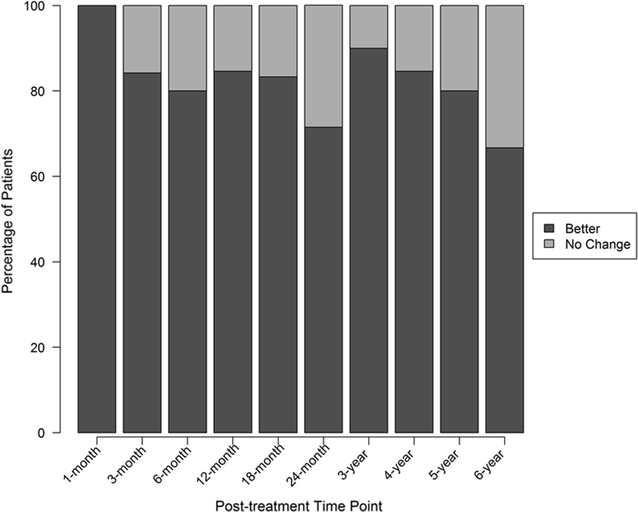
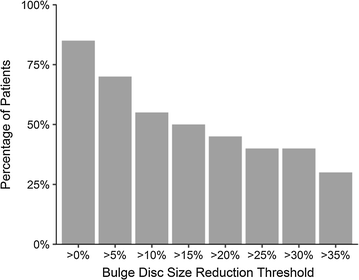
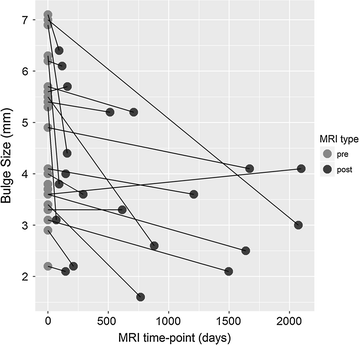
Results: Three patients reported pain related to procedure that resolved. There were no serious adverse events (i.e. death, infection, or tumor) associated with the procedure. NPS change scores relative to baseline were significant at 3, 36, 48, 60, and 72 months post-treatment. The average modified SANE ratings showed a mean improvement of 60% at 3 years post-treatment. FRI post-treatment change score averages exceeded the minimal clinically important difference at all time points except 12 months. Twenty of the patients treated underwent post-treatment MRI and 85% had a reduction in disc bulge size, with an average reduction size of 23% post-treatment.
Conclusions: Patients treated with autologous cultured MSCs for lower back pain with radicular symptoms in the setting of DDD reported minor adverse events and significant improvements in pain, function, and overall subjective improvement through 6 years of follow-up. NCT03011398. A Clinical Registry of Orthobiologics Procedures. https://clinicaltrials.gov/ct2/show/NCT03011398?term=orthobiologics&rank=1.
Keywords: Autologous; Bone marrow; Culture-expanded stem cells; DDD; Degenerative disc disease; Intradiscal injection; MSC; Mesenchymal stem cells; Radicular pain; Regenerative medicine.
Figures
Similar articles
-
Intra-discal injection of autologous, hypoxic cultured bone marrow-derived mesenchymal stem cells in five patients with chronic lower back pain: a long-term safety and feasibility study.J Transl Med. 2016 Sep 1;14(1):253. doi: 10.1186/s12967-016-1015-5. J Transl Med. 2016. PMID: 27585696 Free PMC article.
-
Evaluation of the Effectiveness of Autologous Bone Marrow Mesenchymal Stem Cells in the Treatment of Chronic Low Back Pain Due to Severe Lumbar Spinal Degeneration: A 12-Month, Open-Label, Prospective Controlled Trial.Pain Physician. 2022 Mar;25(2):193-207. Pain Physician. 2022. PMID: 35322978 Clinical Trial.
-
Intervertebral disc repair by autologous mesenchymal bone marrow cells: a pilot study.Transplantation. 2011 Oct 15;92(7):822-8. doi: 10.1097/TP.0b013e3182298a15. Transplantation. 2011. PMID: 21792091 Clinical Trial.
-
Autogenic mesenchymal stem cells for intervertebral disc regeneration.Int Orthop. 2019 Apr;43(4):1027-1036. doi: 10.1007/s00264-018-4218-y. Epub 2018 Nov 10. Int Orthop. 2019. PMID: 30415465
-
Extreme lateral lumbar disc herniation in a 12-year child: case report and review of the literature.Eur Spine J. 2010 Jul;19 Suppl 2(Suppl 2):S197-9. doi: 10.1007/s00586-010-1354-5. Epub 2010 Mar 10. Eur Spine J. 2010. PMID: 20221778 Free PMC article. Review.
Cited by
-
The Analgesic Efficacy of Intradiscal Injection of Bone Marrow Aspirate Concentrate and Culture-Expanded Bone Marrow Mesenchymal Stromal Cells in Discogenic Pain: A Systematic Review.J Pain Res. 2022 Oct 20;15:3299-3318. doi: 10.2147/JPR.S373345. eCollection 2022. J Pain Res. 2022. PMID: 36299501 Free PMC article. Review.
-
Biomaterials and Cell-Based Regenerative Therapies for Intervertebral Disc Degeneration with a Focus on Biological and Biomechanical Functional Repair: Targeting Treatments for Disc Herniation.Cells. 2022 Feb 9;11(4):602. doi: 10.3390/cells11040602. Cells. 2022. PMID: 35203253 Free PMC article. Review.
-
Preclinical to clinical translation for intervertebral disc repair: Effects of species-specific scale, metabolism, and matrix synthesis rates on cell-based regeneration.JOR Spine. 2023 Sep 7;6(3):e1279. doi: 10.1002/jsp2.1279. eCollection 2023 Sep. JOR Spine. 2023. PMID: 37780829 Free PMC article.
-
Mesenchymal stem cells can improve discogenic pain in patients with intervertebral disc degeneration: a systematic review and meta-analysis.Front Bioeng Biotechnol. 2023 Jun 16;11:1155357. doi: 10.3389/fbioe.2023.1155357. eCollection 2023. Front Bioeng Biotechnol. 2023. PMID: 37397969 Free PMC article.
-
The role of microenvironment in stem cell-based regeneration of intervertebral disc.Front Bioeng Biotechnol. 2022 Aug 9;10:968862. doi: 10.3389/fbioe.2022.968862. eCollection 2022. Front Bioeng Biotechnol. 2022. PMID: 36017350 Free PMC article. Review.
References
-
- Frank JW, Brooker AS, DeMaio SE, Kerr MS, Maetzel A, Shannon HS, et al. Disability resulting from occupational low back pain. Part I: what do we know about primary prevention? A review of the scientific evidence on prevention before disability begins. Spine. 1996;21:2908–2917. doi: 10.1097/00007632-199612150-00024. - DOI - PubMed
-
- Manchikanti L, Singh V, Datta S, Cohen SP, Hirsch JA, American Society of Interventional Pain Physicians Comprehensive review of epidemiology, scope, and impact of spinal pain. Pain Physician. 2009;12:E35–E70. - PubMed
MeSH terms
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

