Oral vaccination of wildlife using a vaccinia-rabies-glycoprotein recombinant virus vaccine (RABORAL V-RG®): a global review
- PMID: 28938920
- PMCID: PMC5610451
- DOI: 10.1186/s13567-017-0459-9
Oral vaccination of wildlife using a vaccinia-rabies-glycoprotein recombinant virus vaccine (RABORAL V-RG®): a global review
Abstract
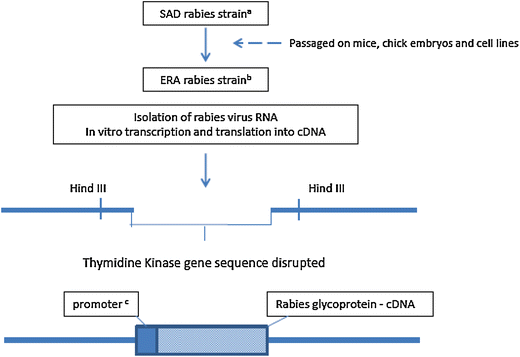
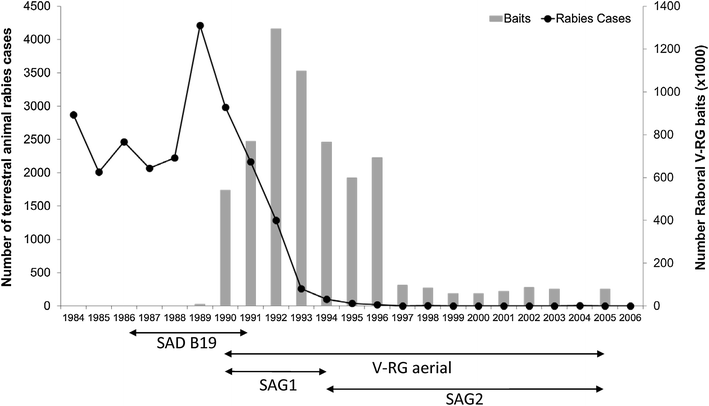
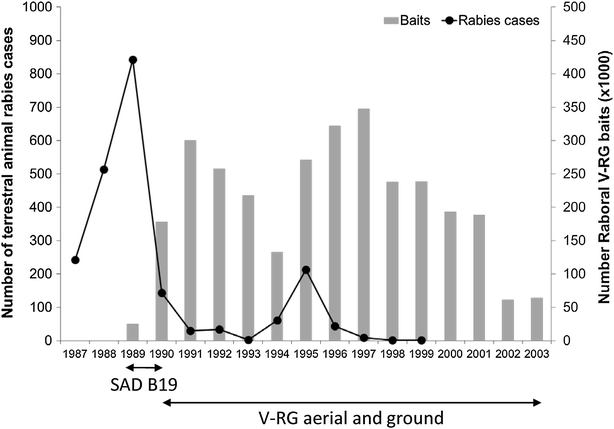
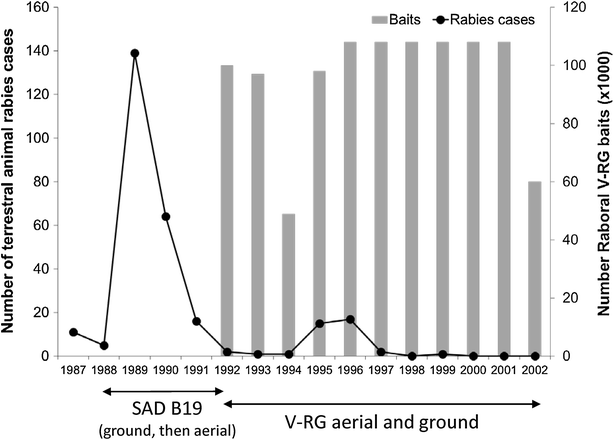
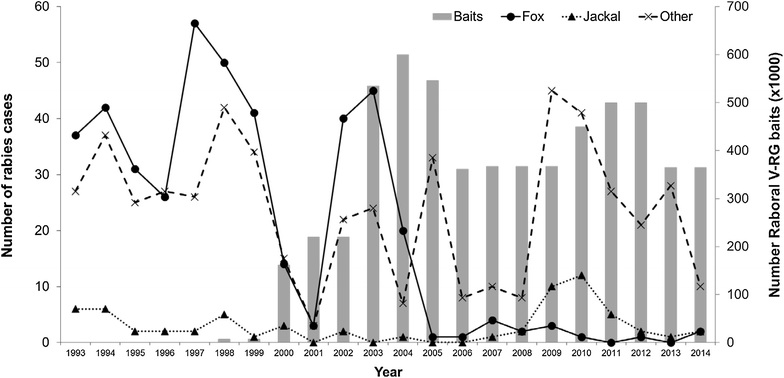
RABORAL V-RG® is an oral rabies vaccine bait that contains an attenuated ("modified-live") recombinant vaccinia virus vector vaccine expressing the rabies virus glycoprotein gene (V-RG). Approximately 250 million doses have been distributed globally since 1987 without any reports of adverse reactions in wildlife or domestic animals since the first licensed recombinant oral rabies vaccine (ORV) was released into the environment to immunize wildlife populations against rabies. V-RG is genetically stable, is not detected in the oral cavity beyond 48 h after ingestion, is not shed by vaccinates into the environment, and has been tested for thermostability under a range of laboratory and field conditions. Safety of V-RG has been evaluated in over 50 vertebrate species, including non-human primates, with no adverse effects observed regardless of route or dose. Immunogenicity and efficacy have been demonstrated under laboratory and field conditions in multiple target species (including fox, raccoon, coyote, skunk, raccoon dog, and jackal). The liquid vaccine is packaged inside edible baits (i.e., RABORAL V-RG, the vaccine-bait product) which are distributed into wildlife habitats for consumption by target species. Field application of RABORAL V-RG has contributed to the elimination of wildlife rabies from three European countries (Belgium, France and Luxembourg) and of the dog/coyote rabies virus variant from the United States of America (USA). An oral rabies vaccination program in west-central Texas has essentially eliminated the gray fox rabies virus variant from Texas with the last case reported in a cow during 2009. A long-term ORV barrier program in the USA using RABORAL V-RG is preventing substantial geographic expansion of the raccoon rabies virus variant. RABORAL V-RG has also been used to control wildlife rabies in Israel for more than a decade. This paper: (1) reviews the development and historical use of RABORAL V-RG; (2) highlights wildlife rabies control programs using the vaccine in multiple species and countries; and (3) discusses current and future challenges faced by programs seeking to control or eliminate wildlife rabies.
Figures


References
-
- National Association of State Public Health Veterinarians, Compendium of Animal Rabies Prevention and Control Committee. Brown CM, Sally Slavinski S, Ettestad P, Sidwa TJ, Sorhage FE. Compendium of animal rabies prevention and control. J Am Vet Med Assoc. 2016;248:505–517. doi: 10.2460/javma.248.5.505. - DOI - PubMed
-
- Esposito J, Brechling K, Baer G, Moss B. Vaccinia virus recombinants expressing rabies virus glycoprotein protect against rabies. Virus Genes. 1987;1:7–21. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

