New kids on the block: The Popeye domain containing (POPDC) protein family acting as a novel class of cAMP effector proteins in striated muscle
- PMID: 28939104
- PMCID: PMC6562197
- DOI: 10.1016/j.cellsig.2017.09.015
New kids on the block: The Popeye domain containing (POPDC) protein family acting as a novel class of cAMP effector proteins in striated muscle
Abstract
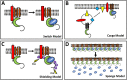
The cyclic 3',5'-adenosine monophosphate (cAMP) signalling pathway constitutes an ancient signal transduction pathway present in prokaryotes and eukaryotes. Previously, it was thought that in eukaryotes three effector proteins mediate cAMP signalling, namely protein kinase A (PKA), exchange factor directly activated by cAMP (EPAC) and the cyclic-nucleotide gated channels. However, recently a novel family of cAMP effector proteins emerged and was termed the Popeye domain containing (POPDC) family, which consists of three members POPDC1, POPDC2 and POPDC3. POPDC proteins are transmembrane proteins, which are abundantly present in striated and smooth muscle cells. POPDC proteins bind cAMP with high affinity comparable to PKA. Presently, their biochemical activity is poorly understood. However, mutational analysis in animal models as well as the disease phenotype observed in patients carrying missense mutations suggests that POPDC proteins are acting by modulating membrane trafficking of interacting proteins. In this review, we will describe the current knowledge about this gene family and also outline the apparent gaps in our understanding of their role in cAMP signalling and beyond.
Keywords: Atrioventricular block; Heart disease; Limb-girdle muscular dystrophy; Popeye domain containing; Protein interaction; Stress-induced bradycardia; cAMP signalling.
Copyright © 2017 The Authors. Published by Elsevier Inc. All rights reserved.
Figures
References
-
- Berthet J., Rall T.W., Sutherland E.W. The relationship of epinephrine and glucagon to liver phosphorylase. IV. Effect of epinephrine and glucagon on the reactivation of phosphorylase in liver homogenates. J. Biol. Chem. 1957;224(1):463–475. - PubMed
-
- El-Armouche A., Eschenhagen T. Beta-adrenergic stimulation and myocardial function in the failing heart. Heart Fail. Rev. 2009;14(4):225–241. - PubMed
-
- Communal C., Singh K., Sawyer D.B., Colucci W.S. Opposing effects of beta(1)- and beta(2)-adrenergic receptors on cardiac myocyte apoptosis: role of a pertussis toxin-sensitive G protein. Circulation. 1999;100(22):2210–2212. - PubMed
-
- Nikolaev V.O., Bunemann M., Schmitteckert E., Lohse M.J., Engelhardt S. Cyclic AMP imaging in adult cardiac myocytes reveals far-reaching beta1-adrenergic but locally confined beta2-adrenergic receptor-mediated signaling. Circ. Res. 2006;99(10):1084–1091. - PubMed
-
- Nikolaev V.O., Moshkov A., Lyon A.R., Miragoli M., Novak P., Paur H., Lohse M.J., Korchev Y.E., Harding S.E., Gorelik J. Beta2-adrenergic receptor redistribution in heart failure changes cAMP compartmentation. Science. 2010;327(5973):1653–1657. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

