α-Ketoglutarate prevents skeletal muscle protein degradation and muscle atrophy through PHD3/ADRB2 pathway
- PMID: 28939592
- PMCID: PMC6266637
- DOI: 10.1096/fj.201700670R
α-Ketoglutarate prevents skeletal muscle protein degradation and muscle atrophy through PHD3/ADRB2 pathway
Erratum in
-
Correction to "α-Ketoglutarate prevents skeletal muscle protein degradation and muscle atrophy through PHD3/ADRB2 pathway".FASEB J. 2024 Jun 15;38(11):e23746. doi: 10.1096/fj.202401222. FASEB J. 2024. PMID: 38855931 No abstract available.
-
Correction to "α-Ketoglutarate prevents skeletal muscle protein degradation and muscle atrophy through PHD3/ADRB2 pathway".FASEB J. 2024 Jul 15;38(13):e23773. doi: 10.1096/fj.202401377. FASEB J. 2024. PMID: 38925566 No abstract available.
Abstract
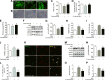
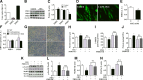
Skeletal muscle atrophy due to excessive protein degradation is the main cause for muscle dysfunction, fatigue, and weakening of athletic ability. Endurance exercise is effective to attenuate muscle atrophy, but the underlying mechanism has not been fully investigated. α-Ketoglutarate (AKG) is a key intermediate of tricarboxylic acid cycle, which is generated during endurance exercise. Here, we demonstrated that AKG effectively attenuated corticosterone-induced protein degradation and rescued the muscle atrophy and dysfunction in a Duchenne muscular dystrophy mouse model. Interestingly, AKG also inhibited the expression of proline hydroxylase 3 (PHD3), one of the important oxidoreductases expressed under hypoxic conditions. Subsequently, we identified the β2 adrenergic receptor (ADRB2) as a downstream target for PHD3. We found AKG inhibited PHD3/ADRB2 interaction and therefore increased the stability of ADRB2. In addition, combining pharmacologic and genetic approaches, we showed that AKG rescues skeletal muscle atrophy and protein degradation through a PHD3/ADRB2 mediated mechanism. Taken together, these data reveal a mechanism for inhibitory effects of AKG on muscle atrophy and protein degradation. These findings not only provide a molecular basis for the potential use of exercise-generated metabolite AKG in muscle atrophy treatment, but also identify PHD3 as a potential target for the development of therapies for muscle wasting.-Cai, X., Yuan, Y., Liao, Z., Xing, K., Zhu, C., Xu, Y., Yu, L., Wang, L., Wang, S., Zhu, X., Gao, P., Zhang, Y., Jiang, Q., Xu, P., Shu, G. α-Ketoglutarate prevents skeletal muscle protein degradation and muscle atrophy through PHD3/ADRB2 pathway.
Keywords: Duchenne muscular dystrophy; metabolism; metabolite; tricarboxylic acid cycle.
© FASEB.
Conflict of interest statement
This work was supported by the National Basic Research Program of China (Grant 2013CB127306 to G.S.), National Key Point Research and Invention Program (Grant 2016YFD0501205 to G.S.), Training Program for Outstanding Young Teachers in the Universities of Guangdong Province (G.S.), National Natural Science Foundation of China (Grants 31572480 to G.S. and 31472105 to Q.J.), and the U.S. National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases (Grant K99DK107008 to P.X.). The authors declare no conflicts of interest.
Figures




References
-
- Malicdan M. C., Noguchi S., Nonaka I., Saftig P., Nishino I. (2008) Lysosomal myopathies: an excessive build-up in autophagosomes is too much to handle. Neuromuscul. Disord. 18, 521–529 - PubMed
-
- Stephens N. A., Gallagher I. J., Rooyackers O., Skipworth R. J., Tan B. H., Marstrand T., Ross J. A., Guttridge D. C., Lundell L., Fearon K. C., Timmons J. A. (2010) Using transcriptomics to identify and validate novel biomarkers of human skeletal muscle cancer cachexia. Genome Med. 2, 1. - PMC - PubMed
-
- Verdijk L. B., Dirks M. L., Snijders T., Prompers J. J., Beelen M., Jonkers R. A., Thijssen D. H., Hopman M. T., Van Loon L. J. (2012) Reduced satellite cell numbers with spinal cord injury and aging in humans. Med. Sci. Sports Exerc. 44, 2322–2330 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

