Coadministration of the Campylobacter jejuni N-Glycan-Based Vaccine with Probiotics Improves Vaccine Performance in Broiler Chickens
- PMID: 28939610
- PMCID: PMC5691412
- DOI: 10.1128/AEM.01523-17
Coadministration of the Campylobacter jejuni N-Glycan-Based Vaccine with Probiotics Improves Vaccine Performance in Broiler Chickens
Abstract
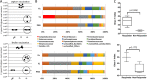
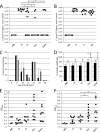
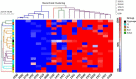
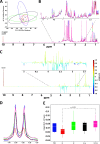
Source attribution studies report that the consumption of contaminated poultry is the primary source for acquiring human campylobacteriosis. Oral administration of an engineered Escherichia coli strain expressing the Campylobacter jejuni N-glycan reduces bacterial colonization in specific-pathogen-free leghorn chickens, but only a fraction of birds respond to vaccination. Optimization of the vaccine for commercial broiler chickens has great potential to prevent the entry of the pathogen into the food chain. Here, we tested the same vaccination approach in broiler chickens and observed similar efficacies in pathogen load reduction, stimulation of the host IgY response, the lack of C. jejuni resistance development, uniformity in microbial gut composition, and the bimodal response to treatment. Gut microbiota analysis of leghorn and broiler vaccine responders identified one member of Clostridiales cluster XIVa, Anaerosporobacter mobilis, that was significantly more abundant in responder birds. In broiler chickens, coadministration of the live vaccine with A. mobilis or Lactobacillus reuteri, a commonly used probiotic, resulted in increased vaccine efficacy, antibody responses, and weight gain. To investigate whether the responder-nonresponder effect was due to the selection of a C. jejuni "supercolonizer mutant" with altered phase-variable genes, we analyzed all poly(G)-containing loci of the input strain compared to nonresponder colony isolates and found no evidence of phase state selection. However, untargeted nuclear magnetic resonance (NMR)-based metabolomics identified a potential biomarker negatively correlated with C. jejuni colonization levels that is possibly linked to increased microbial diversity in this subgroup. The comprehensive methods used to examine the bimodality of the vaccine response provide several opportunities to improve the C. jejuni vaccine and the efficacy of any vaccination strategy.IMPORTANCECampylobacter jejuni is a common cause of human diarrheal disease worldwide and is listed by the World Health Organization as a high-priority pathogen. C. jejuni infection typically occurs through the ingestion of contaminated chicken meat, so many efforts are targeted at reducing C. jejuni levels at the source. We previously developed a vaccine that reduces C. jejuni levels in egg-laying chickens. In this study, we improved vaccine performance in meat birds by supplementing the vaccine with probiotics. In addition, we demonstrated that C. jejuni colonization levels in chickens are negatively correlated with the abundance of clostridia, another group of common gut microbes. We describe new methods for vaccine optimization that will assist in improving the C. jejuni vaccine and other vaccines under development.
Keywords: Campylobacter; glycoengineering; metabolomics; poultry; probiotics; vaccine.
Copyright © 2017 American Society for Microbiology.
Figures

Similar articles
-
Engineering the Campylobacter jejuni N-glycan to create an effective chicken vaccine.Sci Rep. 2016 May 25;6:26511. doi: 10.1038/srep26511. Sci Rep. 2016. PMID: 27221144 Free PMC article.
-
Oral administration of PLGA-encapsulated CpG ODN and Campylobacter jejuni lysate reduces cecal colonization by Campylobacter jejuni in chickens.Vaccine. 2018 Jan 8;36(3):388-394. doi: 10.1016/j.vaccine.2017.11.073. Epub 2017 Dec 7. Vaccine. 2018. PMID: 29223488
-
Evaluation of live-attenuated Salmonella vaccines expressing Campylobacter antigens for control of C. jejuni in poultry.Vaccine. 2010 Jan 22;28(4):1094-105. doi: 10.1016/j.vaccine.2009.10.018. Epub 2009 Oct 22. Vaccine. 2010. PMID: 19853682
-
Design and data analysis of experimental trials to test vaccine candidates against zoonotic pathogens in animals: the case of a clinical trial against campylobacter in broilers.Expert Rev Vaccines. 2012 Oct;11(10):1179-88. doi: 10.1586/erv.12.98. Expert Rev Vaccines. 2012. PMID: 23176651 Review.
-
Campylobacter control in poultry by current intervention measures ineffective: urgent need for intensified fundamental research.Vet Microbiol. 2011 Sep 28;152(3-4):219-28. doi: 10.1016/j.vetmic.2011.03.010. Epub 2011 Mar 16. Vet Microbiol. 2011. PMID: 21482043 Review.
Cited by
-
Improving Chicken Responses to Glycoconjugate Vaccination Against Campylobacter jejuni.Front Microbiol. 2021 Nov 16;12:734526. doi: 10.3389/fmicb.2021.734526. eCollection 2021. Front Microbiol. 2021. PMID: 34867850 Free PMC article.
-
Update on Campylobacter vaccine development.Hum Vaccin Immunother. 2019;15(6):1389-1400. doi: 10.1080/21645515.2018.1528410. Epub 2018 Oct 17. Hum Vaccin Immunother. 2019. PMID: 30252591 Free PMC article. Review.
-
The Role of Nutraceuticals and Phytonutrients in Chickens' Gastrointestinal Diseases.Animals (Basel). 2022 Mar 31;12(7):892. doi: 10.3390/ani12070892. Animals (Basel). 2022. PMID: 35405880 Free PMC article. Review.
-
Immunological and pathobiological characteristics of a novel live Salmonella Typhimurium-vectored Campylobacter vaccine candidate for layer chickens.Front Vet Sci. 2025 Mar 21;12:1518231. doi: 10.3389/fvets.2025.1518231. eCollection 2025. Front Vet Sci. 2025. PMID: 40191081 Free PMC article.
-
How to employ metabolomic analysis to research on functions of prebiotics and probiotics in poultry gut health?Front Microbiol. 2022 Nov 14;13:1040434. doi: 10.3389/fmicb.2022.1040434. eCollection 2022. Front Microbiol. 2022. PMID: 36452931 Free PMC article. Review.
References
-
- Amour C, Gratz J, Mduma E, Svensen E, Rogawski ET, McGrath M, Seidman JC, McCormick BJ, Shrestha S, Samie A, Mahfuz M, Qureshi S, Hotwani A, Babji S, Trigoso DR, Lima AA, Bodhidatta L, Bessong P, Ahmed T, Shakoor S, Kang G, Kosek M, Guerrant RL, Lang D, Gottlieb M, Houpt ER, Platts-Mills JA, Etiology. Risk Factors, and Interactions of Enteric Infections and Malnutrition and the Consequences for Child Health and Development Project Network Investigators. 2016. Epidemiology and impact of Campylobacter infection in children in 8 low-resource settings: results from the MAL-ED study. Clin Infect Dis 63:1171–1179. - PMC - PubMed
-
- European Food Safety Authority, European Centre for Disease Prevention and Control. 2015. The European Union summary report on trends and sources of zoonoses, zoonotic agents and food-borne outbreaks in 2013. EFSA J 13:3991. doi:10.2903/j.efsa.2015.3991. - DOI
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

