Hepatoma-intrinsic CCRK inhibition diminishes myeloid-derived suppressor cell immunosuppression and enhances immune-checkpoint blockade efficacy
- PMID: 28939663
- PMCID: PMC5961939
- DOI: 10.1136/gutjnl-2017-314032
Hepatoma-intrinsic CCRK inhibition diminishes myeloid-derived suppressor cell immunosuppression and enhances immune-checkpoint blockade efficacy
Abstract
Objective: Myeloid-derived suppressor cells (MDSCs) contribute to tumour immunosuppressive microenvironment and immune-checkpoint blockade resistance. Emerging evidence highlights the pivotal functions of cyclin-dependent kinases (CDKs) in tumour immunity. Here we elucidated the role of tumour-intrinsic CDK20, or cell cycle-related kinase (CCRK) on immunosuppression in hepatocellular carcinoma (HCC).
Design: Immunosuppression of MDSCs derived from patients with HCC and relationship with CCRK were determined by flow cytometry, expression analyses and co-culture systems. Mechanistic studies were also conducted in liver-specific CCRK-inducible transgenic (TG) mice and Hepa1-6 orthotopic HCC models using CRISPR/Cas9-mediated Ccrk depletion and liver-targeted nanoparticles for interleukin (IL) 6 trapping. Tumorigenicity and immunophenotype were assessed on single or combined antiprogrammed death-1-ligand 1 (PD-L1) therapy.
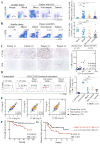
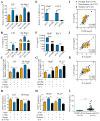
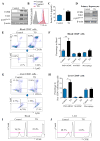
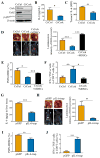
Results: Tumour-infiltrating CD11b+CD33+HLA-DR- MDSCs from patients with HCC potently inhibited autologous CD8+T cell proliferation. Concordant overexpression of CCRK and MDSC markers (CD11b/CD33) positively correlated with poorer survival rates. Hepatocellular CCRK stimulated immunosuppressive CD11b+CD33+HLA-DR- MDSC expansion from human peripheral blood mononuclear cells through upregulating IL-6. Mechanistically, CCRK activated nuclear factor-κB (NF-κB) via enhancer of zeste homolog 2 (EZH2) and facilitated NF-κB-EZH2 co-binding to IL-6 promoter. Hepatic CCRK induction in TG mice activated the EZH2/NF-κB/IL-6 cascade, leading to accumulation of polymorphonuclear (PMN) MDSCs with potent T cell suppressive activity. In contrast, inhibiting tumorous Ccrk or hepatic IL-6 increased interferon γ+tumour necrosis factor-α+CD8+ T cell infiltration and impaired tumorigenicity, which was rescued by restoring PMN-MDSCs. Notably, tumorous Ccrk depletion upregulated PD-L1 expression and increased intratumorous CD8+ T cells, thus enhancing PD-L1 blockade efficacy to eradicate HCC.
Conclusion: Our results delineate an immunosuppressive mechanism of the hepatoma-intrinsic CCRK signalling and highlight an overexpressed kinase target whose inhibition might empower HCC immunotherapy.
Keywords: Cellular Immunology; Hepatocellular Carcinoma; Immunotherapy.
© Article author(s) (or their employer(s) unless otherwise stated in the text of the article) 2018. All rights reserved. No commercial use is permitted unless otherwise expressly granted.
Conflict of interest statement
Competing interests: None declared.
Figures



Comment in
-
CDK20 inhibition and immune checkpoint blockade: bringing cancer biology and tumour immunology together to develop novel treatment options for HCC.Gut. 2018 May;67(5):783-784. doi: 10.1136/gutjnl-2017-315091. Epub 2017 Oct 24. Gut. 2018. PMID: 29066575 Free PMC article. No abstract available.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous
