PD-1 Blockade Promotes Epitope Spreading in Anticancer CD8+ T Cell Responses by Preventing Fratricidal Death of Subdominant Clones To Relieve Immunodomination
- PMID: 28939757
- PMCID: PMC5731479
- DOI: 10.4049/jimmunol.1700643
PD-1 Blockade Promotes Epitope Spreading in Anticancer CD8+ T Cell Responses by Preventing Fratricidal Death of Subdominant Clones To Relieve Immunodomination
Abstract
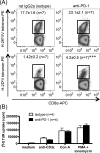
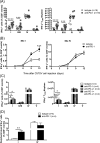
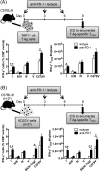
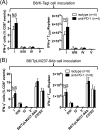
The interactions between programmed death-1 (PD-1) and its ligands hamper tumor-specific CD8+ T cell (TCD8) responses, and PD-1-based "checkpoint inhibitors" have shown promise in certain cancers, thus revitalizing interest in immunotherapy. PD-1-targeted therapies reverse TCD8 exhaustion/anergy. However, whether they alter the epitope breadth of TCD8 responses remains unclear. This is an important question because subdominant TCD8 are more likely than immunodominant clones to escape tolerance mechanisms and may contribute to protective anticancer immunity. We have addressed this question in an in vivo model of TCD8 responses to well-defined epitopes of a clinically relevant oncoprotein, large T Ag. We found that unlike other coinhibitory molecules (CTLA-4, LAG-3, TIM-3), PD-1 was highly expressed by subdominant TCD8, which correlated with their propensity to favorably respond to PD-1/PD-1 ligand-1 (PD-L1)-blocking Abs. PD-1 blockade increased the size of subdominant TCD8 clones at the peak of their primary response, and it also sustained their presence, thus giving rise to an enlarged memory pool. The expanded population was fully functional as judged by IFN-γ production and MHC class I-restricted cytotoxicity. The selective increase in subdominant TCD8 clonal size was due to their enhanced survival, not proliferation. Further mechanistic studies utilizing peptide-pulsed dendritic cells, recombinant vaccinia viruses encoding full-length T Ag or epitope mingenes, and tumor cells expressing T Ag variants revealed that anti-PD-1 invigorates subdominant TCD8 responses by relieving their lysis-dependent suppression by immunodominant TCD8 To our knowledge, our work constitutes the first report that interfering with PD-1 signaling potentiates epitope spreading in tumor-specific responses, a finding with clear implications for cancer immunotherapy and vaccination.
Copyright © 2017 by The American Association of Immunologists, Inc.
Figures





Similar articles
-
PD-1/PD-L1 co-inhibition shapes anticancer T cell immunodominance: facing the consequences of an immunological ménage à trois.Cancer Immunol Immunother. 2018 Nov;67(11):1669-1672. doi: 10.1007/s00262-018-2231-z. Epub 2018 Aug 21. Cancer Immunol Immunother. 2018. PMID: 30132082 Free PMC article.
-
Discordant rearrangement of primary and anamnestic CD8+ T cell responses to influenza A viral epitopes upon exposure to bacterial superantigens: Implications for prophylactic vaccination, heterosubtypic immunity and superinfections.PLoS Pathog. 2020 May 20;16(5):e1008393. doi: 10.1371/journal.ppat.1008393. eCollection 2020 May. PLoS Pathog. 2020. PMID: 32433711 Free PMC article.
-
CD4+ T-cell epitope-based heterologous prime-boost vaccination potentiates anti-tumor immunity and PD-1/PD-L1 immunotherapy.J Immunother Cancer. 2022 May;10(5):e004022. doi: 10.1136/jitc-2021-004022. J Immunother Cancer. 2022. PMID: 35580929 Free PMC article.
-
Immunodominance in major histocompatibility complex class I-restricted T lymphocyte responses.Annu Rev Immunol. 1999;17:51-88. doi: 10.1146/annurev.immunol.17.1.51. Annu Rev Immunol. 1999. PMID: 10358753 Review.
-
Revealing factors determining immunodominant responses against dominant epitopes.Immunogenetics. 2020 Feb;72(1-2):109-118. doi: 10.1007/s00251-019-01134-9. Epub 2019 Dec 6. Immunogenetics. 2020. PMID: 31811313 Free PMC article. Review.
Cited by
-
Neurologic Toxicity of Immune Checkpoint Inhibitors: A Review of Literature.Front Pharmacol. 2022 Feb 14;13:774170. doi: 10.3389/fphar.2022.774170. eCollection 2022. Front Pharmacol. 2022. PMID: 35237154 Free PMC article. Review.
-
MEK inhibition enhances oncolytic virus immunotherapy through increased tumor cell killing and T cell activation.Sci Transl Med. 2018 Dec 12;10(471):eaau0417. doi: 10.1126/scitranslmed.aau0417. Sci Transl Med. 2018. PMID: 30541787 Free PMC article.
-
PD-1/PD-L1 co-inhibition shapes anticancer T cell immunodominance: facing the consequences of an immunological ménage à trois.Cancer Immunol Immunother. 2018 Nov;67(11):1669-1672. doi: 10.1007/s00262-018-2231-z. Epub 2018 Aug 21. Cancer Immunol Immunother. 2018. PMID: 30132082 Free PMC article.
-
Immune checkpoint receptors: homeostatic regulators of immunity.Hepatol Int. 2018 May;12(3):223-236. doi: 10.1007/s12072-018-9867-9. Epub 2018 May 8. Hepatol Int. 2018. PMID: 29740793 Free PMC article. Review.
-
Bispecific Antibodies in Prostate Cancer Therapy: Current Status and Perspectives.Cancers (Basel). 2021 Feb 1;13(3):549. doi: 10.3390/cancers13030549. Cancers (Basel). 2021. PMID: 33535627 Free PMC article. Review.
References
-
- Gallimore A, Glithero A, Godkin A, Tissot AC, Pluckthun A, Elliott T, Hengartner H, Zinkernagel R. Induction and exhaustion of lymphocytic choriomeningitis virus-specific cytotoxic T lymphocytes visualized using soluble tetrameric major histocompatibility complex class I-peptide complexes. J Exp Med. 1998;187:1383–1393. - PMC - PubMed
-
- Sharma P, Allison JP. The future of immune checkpoint therapy. Science. 2015;348:56–61. - PubMed
-
- Hoffmann M, Pantazis N, Martin GE, Hickling S, Hurst J, Meyerowitz J, Willberg CB, Robinson N, Brown H, Fisher M, Kinloch S, Babiker A, Weber J, Nwokolo N, Fox J, Fidler S, Phillips R, Frater J Spartac, and C. Investigators. Exhaustion of Activated CD8 T Cells Predicts Disease Progression in Primary HIV-1 Infection. PLoS Pathog. 2016;12:e1005661. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

