Estrogenic vascular effects are diminished by chronological aging
- PMID: 28939871
- PMCID: PMC5610317
- DOI: 10.1038/s41598-017-12153-5
Estrogenic vascular effects are diminished by chronological aging
Abstract
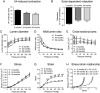
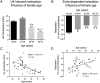
The beneficial role of estrogen in the vascular system may be due, in part, through reduction of peripheral vascular resistance. The use of estrogen therapy to prevent cardiovascular disease in post-menopausal women remains contentious. This study investigated the influence of aging and the menopause on the acute vasodilatory effects of estrogen using ex vivo human and murine resistance arteries. Vessels were obtained from young (2.9 ± 0.1 months) and aged (24.2 ± 0.1 and 28.9 ± 0.3 months) female mice and pre- (42.3 ± 0.5 years) and post-menopausal (61.9 ± 0.9 years) women. Aging was associated with profound structural alterations of murine uterine arteries, including the occurrence of outward hypertrophic remodeling and increased stiffness. Endothelial and smooth muscle function were diminished in uterine (and tail) arteries from aged mice and post-menopausal women. The acute vasodilatory effects of 17β-estradiol (non-specific estrogen receptor (ER) agonist), PPT (ERα-specific agonist) and DPN (ERβ-specific agonist) on resistance arteries were attenuated by aging and the menopause. However, the impairment of estrogenic relaxation was evident after the occurrence of age-related endothelial dysfunction and diminished distensibility. The data indicate, therefore, that chronological resistance arterial aging is a prominent factor leading to weakened vasodilatory action of estrogenic compounds.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures




References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

