Identification of a specific agonist of human TAS2R14 from Radix Bupleuri through virtual screening, functional evaluation and binding studies
- PMID: 28939897
- PMCID: PMC5610306
- DOI: 10.1038/s41598-017-11720-0
Identification of a specific agonist of human TAS2R14 from Radix Bupleuri through virtual screening, functional evaluation and binding studies
Abstract
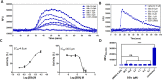
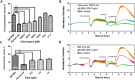
Bitter taste receptors (TAS2Rs) have attracted a great deal of interest because of their recently described bronchodilator and anti-inflammatory properties. The aim of this study was to identify natural direct TAS2R14 agonists from Radix Bupleuri that can inhibit mast cell degranulation. A ligand-based virtual screening was conducted on a library of chemicals contained in compositions of Radix Bupleuri, and these analyses were followed by cell-based functional validation through a HEK293-TAS2R14-G16gust44 cell line and IgE-induced mast cell degranulation assays, respectively. Saikosaponin b (SSb) was confirmed for the first time to be a specific agonist of TAS2R14 and had an EC50 value of 4.9 μM. A molecular docking study showed that SSb could directly bind to a TAS2R14 model through H-bond interactions with Arg160, Ser170 and Glu259. Moreover, SSb showed the ability to inhibit IgE-induced mast cell degranulation, as measured with a β-hexosaminidase release model and real-time cell analysis (RTCA). In a cytotoxicity bioassay, SSb showed no significant cytotoxicity to HEK293 cells within 24 hours. This study demonstrated that SSb is a direct TAS2R14 agonist that inhibit IgE-induced mast cell degranulation. Although the target and in vitro bioactivity of SSb were revealed in this study, it still need in vivo study to further verify the anti-asthma activity of SSb.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures
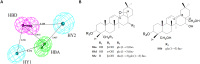
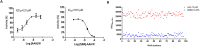


Similar articles
-
Saikosaponin D from Radix Bupleuri suppresses triple-negative breast cancer cell growth by targeting β-catenin signaling.Biomed Pharmacother. 2018 Dec;108:724-733. doi: 10.1016/j.biopha.2018.09.038. Epub 2018 Sep 21. Biomed Pharmacother. 2018. PMID: 30248540
-
Reengineering the ligand sensitivity of the broadly tuned human bitter taste receptor TAS2R14.Biochim Biophys Acta Gen Subj. 2018 Oct;1862(10):2162-2173. doi: 10.1016/j.bbagen.2018.07.009. Epub 2018 Jul 19. Biochim Biophys Acta Gen Subj. 2018. PMID: 30009876
-
Saikosaponin A inhibits compound 48/80-induced pseudo-allergy via the Mrgprx2 pathway in vitro and in vivo.Biochem Pharmacol. 2018 Feb;148:147-154. doi: 10.1016/j.bcp.2017.12.017. Epub 2017 Dec 21. Biochem Pharmacol. 2018. PMID: 29274317
-
Saikosaponin D: review on the antitumour effects, toxicity and pharmacokinetics.Pharm Biol. 2021 Dec;59(1):1480-1489. doi: 10.1080/13880209.2021.1992448. Pharm Biol. 2021. PMID: 34714209 Free PMC article. Review.
-
Pharmacological Effects and Chemical Constituents of Bupleurum.Mini Rev Med Chem. 2019;19(1):34-55. doi: 10.2174/1871520618666180628155931. Mini Rev Med Chem. 2019. PMID: 29956627 Review.
Cited by
-
Bitter Phytochemicals as Novel Candidates for Skin Disease Treatment.Curr Issues Mol Biol. 2023 Dec 30;46(1):299-326. doi: 10.3390/cimb46010020. Curr Issues Mol Biol. 2023. PMID: 38248322 Free PMC article. Review.
-
Discovery of a Natural Syk Inhibitor from Chinese Medicine through a Docking-Based Virtual Screening and Biological Assay Study.Molecules. 2018 Nov 28;23(12):3114. doi: 10.3390/molecules23123114. Molecules. 2018. PMID: 30487406 Free PMC article.
-
Biochanin a modulates steroidogenesis and cellular metabolism in human granulosa cells through TAS2Rs activation: a spotlight on ovarian function.Reprod Biol Endocrinol. 2025 Jan 25;23(1):13. doi: 10.1186/s12958-025-01344-9. Reprod Biol Endocrinol. 2025. PMID: 39863886 Free PMC article.
-
Saikosaponin b2 enhances the hepatotargeting effect of anticancer drugs through inhibition of multidrug resistance-associated drug transporters.Life Sci. 2019 Aug 15;231:116557. doi: 10.1016/j.lfs.2019.116557. Epub 2019 Jun 10. Life Sci. 2019. PMID: 31194994 Free PMC article.
-
DNA copy number variations in children with vesicoureteral reflux and urinary tract infections.PLoS One. 2019 Aug 12;14(8):e0220617. doi: 10.1371/journal.pone.0220617. eCollection 2019. PLoS One. 2019. PMID: 31404082 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

