Pore formation in lipid membrane I: Continuous reversible trajectory from intact bilayer through hydrophobic defect to transversal pore
- PMID: 28939906
- PMCID: PMC5610326
- DOI: 10.1038/s41598-017-12127-7
Pore formation in lipid membrane I: Continuous reversible trajectory from intact bilayer through hydrophobic defect to transversal pore
Abstract
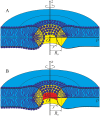
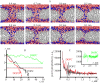
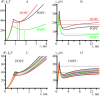
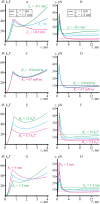
Lipid membranes serve as effective barriers allowing cells to maintain internal composition differing from that of extracellular medium. Membrane permeation, both natural and artificial, can take place via appearance of transversal pores. The rearrangements of lipids leading to pore formation in the intact membrane are not yet understood in details. We applied continuum elasticity theory to obtain continuous trajectory of pore formation and closure, and analyzed molecular dynamics trajectories of pre-formed pore reseal. We hypothesized that a transversal pore is preceded by a hydrophobic defect: intermediate structure spanning through the membrane, the side walls of which are partially aligned by lipid tails. This prediction was confirmed by our molecular dynamics simulations. Conversion of the hydrophobic defect into the hydrophilic pore required surmounting some energy barrier. A metastable state was found for the hydrophilic pore at the radius of a few nanometers. The dependence of the energy on radius was approximately quadratic for hydrophobic defect and small hydrophilic pore, while for large radii it depended on the radius linearly. The pore energy related to its perimeter, line tension, thus depends of the pore radius. Calculated values of the line tension for large pores were in quantitative agreement with available experimental data.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures


Similar articles
-
The importance of membrane defects-lessons from simulations.Acc Chem Res. 2014 Aug 19;47(8):2244-51. doi: 10.1021/ar4002729. Epub 2014 Jun 3. Acc Chem Res. 2014. PMID: 24892900
-
Pore formation in lipid membrane II: Energy landscape under external stress.Sci Rep. 2017 Oct 2;7(1):12509. doi: 10.1038/s41598-017-12749-x. Sci Rep. 2017. PMID: 28970526 Free PMC article.
-
Pore nucleation in mechanically stretched bilayer membranes.J Chem Phys. 2005 Oct 15;123(15):154701. doi: 10.1063/1.2060666. J Chem Phys. 2005. PMID: 16252963
-
A lipocentric view of peptide-induced pores.Eur Biophys J. 2011 Apr;40(4):399-415. doi: 10.1007/s00249-011-0693-4. Epub 2011 Mar 26. Eur Biophys J. 2011. PMID: 21442255 Free PMC article. Review.
-
Continuum Models of Membrane Fusion: Evolution of the Theory.Int J Mol Sci. 2020 May 29;21(11):3875. doi: 10.3390/ijms21113875. Int J Mol Sci. 2020. PMID: 32485905 Free PMC article. Review.
Cited by
-
Switching between Successful and Dead-End Intermediates in Membrane Fusion.Int J Mol Sci. 2017 Dec 2;18(12):2598. doi: 10.3390/ijms18122598. Int J Mol Sci. 2017. PMID: 29207481 Free PMC article.
-
Free Energy of Membrane Pore Formation and Stability from Molecular Dynamics Simulations.J Chem Inf Model. 2025 Jan 27;65(2):908-920. doi: 10.1021/acs.jcim.4c01960. Epub 2025 Jan 10. J Chem Inf Model. 2025. PMID: 39792085 Free PMC article.
-
Determination of Elastic Parameters of Lipid Membranes with Molecular Dynamics: A Review of Approaches and Theoretical Aspects.Membranes (Basel). 2022 Nov 16;12(11):1149. doi: 10.3390/membranes12111149. Membranes (Basel). 2022. PMID: 36422141 Free PMC article. Review.
-
Propidium uptake and ATP release in A549 cells share similar transport mechanisms.Biophys J. 2022 May 3;121(9):1593-1609. doi: 10.1016/j.bpj.2022.04.007. Epub 2022 Apr 6. Biophys J. 2022. PMID: 35398020 Free PMC article.
-
Enhanced gene delivery in tumor cells using chemical carriers and mechanical loadings.PLoS One. 2018 Dec 28;13(12):e0209199. doi: 10.1371/journal.pone.0209199. eCollection 2018. PLoS One. 2018. PMID: 30592721 Free PMC article.
References
-
- Chakrabarti R, Wylie DE, Schuster SM. Transfer of monoclonal antibodies into mammalian cells by electroporation. J. Biol. Chem. 1989;264:15494–15500. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

