CIB1 protects against MPTP-induced neurotoxicity through inhibiting ASK1
- PMID: 28939911
- PMCID: PMC5610320
- DOI: 10.1038/s41598-017-12379-3
CIB1 protects against MPTP-induced neurotoxicity through inhibiting ASK1
Abstract
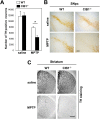
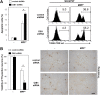
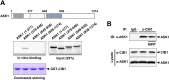
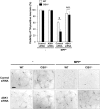
Calcium and integrin binding protein 1 (CIB1) is a calcium-binding protein that was initially identified as a binding partner of platelet integrin αIIb. Although CIB1 has been shown to interact with multiple proteins, its biological function in the brain remains unclear. Here, we show that CIB1 negatively regulates degeneration of dopaminergic neurons in a mouse model of Parkinson's disease using 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP). Genetic deficiency of the CIB1 gene enhances MPTP-induced neurotoxicity in dopaminergic neurons in CIB1-/- mice. Furthermore, RNAi-mediated depletion of CIB1 in primary dopaminergic neurons potentiated 1-methyl-4-phenyl pyrinidium (MPP+)-induced neuronal death. CIB1 physically associated with apoptosis signal-regulating kinase 1 (ASK1) and thereby inhibited the MPP+-induced stimulation of the ASK1-mediated signaling cascade. These findings suggest that CIB1 plays a protective role in MPTP/MPP+-induced neurotoxicity by blocking ASK1-mediated signaling.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures


Similar articles
-
Small peptide inhibitor of JNK3 protects dopaminergic neurons from MPTP induced injury via inhibiting the ASK1-JNK3 signaling pathway.PLoS One. 2015 Apr 9;10(4):e0119204. doi: 10.1371/journal.pone.0119204. eCollection 2015. PLoS One. 2015. PMID: 25856433 Free PMC article.
-
Secalonic acid A protects dopaminergic neurons from 1-methyl-4-phenylpyridinium (MPP⁺)-induced cell death via the mitochondrial apoptotic pathway.Eur J Pharmacol. 2013 Aug 5;713(1-3):58-67. doi: 10.1016/j.ejphar.2013.04.029. Epub 2013 May 9. Eur J Pharmacol. 2013. PMID: 23665112
-
CIB1 functions as a Ca(2+)-sensitive modulator of stress-induced signaling by targeting ASK1.Proc Natl Acad Sci U S A. 2009 Oct 13;106(41):17389-94. doi: 10.1073/pnas.0812259106. Epub 2009 Sep 29. Proc Natl Acad Sci U S A. 2009. PMID: 19805025 Free PMC article.
-
SEA0400, a specific Na+/Ca2+ exchange inhibitor, prevents dopaminergic neurotoxicity in an MPTP mouse model of Parkinson's disease.Neuropharmacology. 2011 Dec;61(8):1441-51. doi: 10.1016/j.neuropharm.2011.08.041. Epub 2011 Sep 2. Neuropharmacology. 2011. PMID: 21903118
-
JNK inhibition as a potential strategy in treating Parkinson's disease.Drug News Perspect. 2004 Dec;17(10):646-54. doi: 10.1358/dnp.2004.17.10.873916. Drug News Perspect. 2004. PMID: 15696229 Review.
Cited by
-
Combined surface functionalization of MSC membrane and PDA inhibits neurotoxicity induced by Fe3O4 in mice based on apoptosis and autophagy through the ASK1/JNK signaling pathway.Aging (Albany NY). 2023 Jul 19;15(14):6933-6949. doi: 10.18632/aging.204884. Epub 2023 Jul 19. Aging (Albany NY). 2023. PMID: 37470690 Free PMC article.
-
The Diverse Functions of the Calcium- and Integrin-Binding Protein Family.Int J Mol Sci. 2025 Feb 28;26(5):2223. doi: 10.3390/ijms26052223. Int J Mol Sci. 2025. PMID: 40076845 Free PMC article. Review.
-
Functions of p38 MAP Kinases in the Central Nervous System.Front Mol Neurosci. 2020 Sep 8;13:570586. doi: 10.3389/fnmol.2020.570586. eCollection 2020. Front Mol Neurosci. 2020. PMID: 33013322 Free PMC article. Review.
-
Application of OpenArray Technology to Assess Changes in the Expression of Functionally Significant Genes in the Substantia Nigra of Mice in a Model of Parkinson's Disease.Genes (Basel). 2023 Dec 12;14(12):2202. doi: 10.3390/genes14122202. Genes (Basel). 2023. PMID: 38137024 Free PMC article.
-
The Function of ASK1 in Sepsis and Stress-Induced Disorders.Int J Mol Sci. 2023 Dec 22;25(1):213. doi: 10.3390/ijms25010213. Int J Mol Sci. 2023. PMID: 38203381 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

