Ketamine-Induced Glutamatergic Mechanisms of Sleep and Wakefulness: Insights for Developing Novel Treatments for Disturbed Sleep and Mood
- PMID: 28939975
- PMCID: PMC5866161
- DOI: 10.1007/164_2017_51
Ketamine-Induced Glutamatergic Mechanisms of Sleep and Wakefulness: Insights for Developing Novel Treatments for Disturbed Sleep and Mood
Abstract
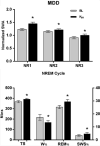
Ketamine, a drug with rapid antidepressant effects and well-described effects on slow wave sleep (SWS), is a useful intervention for investigating sleep-wake mechanisms involved in novel therapeutics. The drug rapidly (within minutes to hours) reduces depressive symptoms in individuals with major depressive disorder (MDD) or bipolar disorder (BD), including those with treatment-resistant depression. Ketamine treatment elevates extracellular glutamate in the prefrontal cortex. Glutamate, in turn, plays a critical role as a proximal element in a ketamine-initiated molecular cascade that increases synaptic strength and plasticity, which ultimately results in rapidly improved mood. In MDD, rapid antidepressant response to ketamine is related to decreased waking as well as increased total sleep, SWS, slow wave activity (SWA), and rapid eye movement (REM) sleep. Ketamine also increases brain-derived neurotrophic factor (BDNF) levels. In individuals with MDD, clinical response to ketamine is predicted by low baseline delta sleep ratio, a measure of deficient early night production of SWS. Notably, there are important differences between MDD and BD that may be related to the effects of diagnosis or of mood stabilizers. Consistent with its effects on clock-associated molecules, ketamine alters the timing and amplitude of circadian activity patterns in rapid responders versus non-responders with MDD, suggesting that it affects mood-dependent central neural circuits. Molecular interactions between sleep homeostasis and clock genes may mediate the rapid and durable elements of clinical response to ketamine and its active metabolite.
Keywords: Brain-derived neurotrophic factor (BDNF); Circadian; Major depressive disorder; Neuroplasticity; Slow wave sleep; Suicidality.
Figures

References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

