Validation of the mIBG skeletal SIOPEN scoring method in two independent high-risk neuroblastoma populations: the SIOPEN/HR-NBL1 and COG-A3973 trials
- PMID: 28940046
- PMCID: PMC5747549
- DOI: 10.1007/s00259-017-3829-7
Validation of the mIBG skeletal SIOPEN scoring method in two independent high-risk neuroblastoma populations: the SIOPEN/HR-NBL1 and COG-A3973 trials
Abstract
Background: Validation of the prognostic value of the SIOPEN mIBG skeletal scoring system in two independent stage 4, mIBG avid, high-risk neuroblastoma populations.
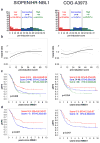
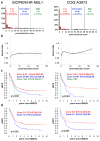
Results: The semi-quantitative SIOPEN score evaluates skeletal meta-iodobenzylguanidine (mIBG) uptake on a 0-6 scale in 12 anatomical regions. Evaluable mIBG scans from 216 COG-A3973 and 341 SIOPEN/HR-NBL1 trial patients were reviewed pre- and post-induction chemotherapy. The prognostic value of skeletal scores for 5-year event free survival (5 yr.-EFS) was tested in the source and validation cohorts. At diagnosis, both cohorts showed a gradual non-linear increase in risk with cumulative scores. Several approaches were explored to test the relationship between score and EFS. Ultimately, a cutoff score of ≤3 was the most useful predictor across trials. A SIOPEN score ≤ 3 pre-induction was found in 15% SIOPEN patients and in 22% of COG patients and increased post-induction to 60% in SIOPEN patients and to 73% in COG patients. Baseline 5 yr.-EFS rates in the SIOPEN/HR-NBL1 cohort for scores ≤3 were 47% ± 7% versus 26% ± 3% for higher scores at diagnosis (p < 0.007) and 36% ± 4% versus 14% ± 4% (p < 0.001) for scores obtained post-induction. The COG-A3973 showed 5 yr.-EFS rates for scores ≤3 of 51% ± 7% versus 34% ± 4% for higher scores (p < 0.001) at diagnosis and 43% ± 5% versus 16% ± 6% (p = 0.004) for post-induction scores. Hazard ratios (HR) significantly favoured patients with scores ≤3 after adjustment for age and MYCN-amplification. Optimal outcomes were recorded in patients who achieved complete skeletal response.
Conclusions: Validation in two independent cohorts confirms the prognostic value of the SIOPEN skeletal score. In particular, patients with an absolute SIOPEN score > 3 after induction have very poor outcomes and should be considered for alternative therapeutic strategies.
Keywords: High-risk neuroblastoma; MIBG; SIOPEN score.
Conflict of interest statement
Figures




References
-
- London WB, Castleberry RP, Matthay KK, Look AT, Seeger RC, Shimada H, et al. Evidence for an age cutoff greater than 365 days for neuroblastoma risk group stratification in the Children's oncology group. J Clin Oncol. 2005;23:6459–65. - PubMed
-
- Moroz V, Machin D, Faldum A, Hero B, Iehara T, Mosseri V, et al. Changes over three decades in outcome and the prognostic influence of age-at-diagnosis in young patients with neuroblastoma: a report from the international neuroblastoma risk group project. Eur J Cancer. 2011;47:561–71. - PubMed
-
- Seeger RC, Brodeur GM, Sather H, Dalton A, Siegel SE, Wong KY, et al. Association of multiple copies of the N-myc oncogene with rapid progression of neuroblastomas. N Engl J Med. 1985;313:1111–6. - PubMed
-
- Shimada H, Stram DO, Chatten J, Joshi VV, Hachitanda Y, Brodeur GM, et al. Identification of subsets of neuroblastomas by combined histopathologic and N-myc analysis. J Natl Cancer Inst. 1995;87:1470–6. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

