Lipid homeostasis and inflammatory activation are disturbed in classically activated macrophages with peroxisomal β-oxidation deficiency
- PMID: 28940384
- PMCID: PMC5795189
- DOI: 10.1111/imm.12844
Lipid homeostasis and inflammatory activation are disturbed in classically activated macrophages with peroxisomal β-oxidation deficiency
Abstract
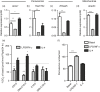
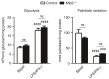
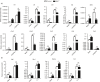
Macrophage activation is characterized by pronounced metabolic adaptation. Classically activated macrophages show decreased rates of mitochondrial fatty acid oxidation and oxidative phosphorylation and acquire a glycolytic state together with their pro-inflammatory phenotype. In contrast, alternatively activated macrophages require oxidative phosphorylation and mitochondrial fatty acid oxidation for their anti-inflammatory function. Although it is evident that mitochondrial metabolism is regulated during macrophage polarization and essential for macrophage function, little is known on the regulation and role of peroxisomal β-oxidation during macrophage activation. In this study, we show that peroxisomal β-oxidation is strongly decreased in classically activated bone-marrow-derived macrophages (BMDM) and mildly induced in alternatively activated BMDM. To examine the role of peroxisomal β-oxidation in macrophages, we used Mfp2-/- BMDM lacking the key enzyme of this pathway. Impairment of peroxisomal β-oxidation in Mfp2-/- BMDM did not cause lipid accumulation but rather an altered distribution of lipid species with very-long-chain fatty acids accumulating in the triglyceride and phospholipid fraction. These lipid alterations in Mfp2-/- macrophages led to decreased inflammatory activation of Mfp2-/- BMDM and peritoneal macrophages evidenced by impaired production of several inflammatory cytokines and chemokines, but did not affect anti-inflammatory polarization. The disturbed inflammatory responses of Mfp2-/- macrophages did not affect immune cell infiltration, as mice with selective elimination of MFP2 from myeloid cells showed normal monocyte and neutrophil influx upon challenge with zymosan. Together, these data demonstrate that peroxisomal β-oxidation is involved in fine-tuning the phenotype of macrophages, probably by influencing the dynamic lipid profile during macrophage polarization.
Keywords: macrophages; metabolism; multifunctional protein 2; peroxisomal β-oxidation.
© 2017 John Wiley & Sons Ltd.
Figures






References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

