Long-term follow-up of clinical trial patients treated for chronic HCV infection with daclatasvir-based regimens
- PMID: 28941023
- PMCID: PMC5947593
- DOI: 10.1111/liv.13596
Long-term follow-up of clinical trial patients treated for chronic HCV infection with daclatasvir-based regimens
Abstract
Background & aims: Daclatasvir has achieved high sustained virologic response (SVR) rates in diverse hepatitis C virus (HCV) populations. This study evaluated the long-term efficacy and safety of daclatasvir-based regimens administered during clinical studies.
Methods: Patients enrolled within 6 months of parent study completion or protocol availability at the study sites. The primary objective was durability of SVR at follow-up Week 12 (SVR12). Secondary objectives included analysing HCV sequences in non-responders or responders who relapsed, and characterization of liver disease progression.
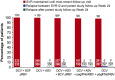
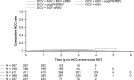
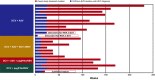
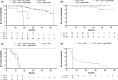
Results: Between 24 February 2012 and 17 July 2015, this study enrolled and began following 1503 recipients of daclatasvir-based regimens (follow-up cut-off, 13 October 2015); 60% were male, 18% aged ≥65 years, 87% had genotype-1a (42%) or -1b (45%) infection, and 18% had cirrhosis. Median follow-up from parent study follow-up Week 12 was 111 (range, 11-246) weeks. 1329/1489 evaluable patients were SVR12 responders; 1316/1329 maintained SVR until their latest visit. Twelve responders relapsed by (n = 9) or after (n = 3) parent study follow-up Week 24; one was reinfected. Relapse occurred in 3/842 (0.4%) and 9/487 (2%) responders treated with interferon-free or interferon-containing regimens, respectively. Hepatic disease progression and new hepatocellular carcinoma were diagnosed in 15 and 23 patients, respectively. Among non-responders, emergent non-structural protein-5A (NS5A) and -3 (NS3) substitutions were replaced by wild-type sequences in 27/157 (17%) and 35/47 (74%) patients, respectively.
Conclusions: SVR12 was durable in 99% of recipients of daclatasvir-based regimens. Hepatic disease progression and new hepatocellular carcinoma were infrequent. Emergent NS5A substitutions persisted longer than NS3 substitutions among non-responders.
Trial registration: ClinicalTrials.gov NCT01492504.
Keywords: chronic hepatitis C virus; daclatasvir; hepatocellular carcinoma; long-term follow-up; sustained virologic response.
© 2017 The Authors. Liver International Published by John Wiley & Sons Ltd.
Figures
References
-
- American Association for the Study of Liver Diseases and the Infectious Diseases Society of America . Recommendations for testing, managing, and treating hepatitis C. http://www hcvguidelines org/full‐report‐view. Accessed September 1, 2017.
-
- European Association for Study of Liver . EASL recommendations on treatment of hepatitis C 2015. J Hepatol. 2015;63:199‐236. - PubMed
-
- Gao M. Antiviral activity and resistance of HCV NS5A replication complex inhibitors. Curr Opin Virol. 2013;3:514‐520. - PubMed
-
- Hezode C, Abergel A, Chas J, et al. Sustained virologic response to daclatasvir and sofosbuvir, with or without ribavirin, among patients in the french daclatasvir ATU programme infected with HCV genotypes‐4, 5, and 6. J Hepatol. 2016;64(suppl):S755.
-
- European Medicines Agency . Daklinza (daclatasvir) summary of product characteristics (updated, 19‐September‐2016).
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

