Reactive Electrophilic OI- Species Evidenced in High-Performance Iridium Oxohydroxide Water Oxidation Electrocatalysts
- PMID: 28941180
- PMCID: PMC5813174
- DOI: 10.1002/cssc.201701291
Reactive Electrophilic OI- Species Evidenced in High-Performance Iridium Oxohydroxide Water Oxidation Electrocatalysts
Abstract
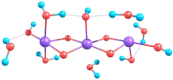
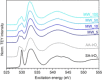
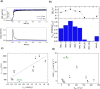
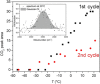
Although quasi-amorphous iridium oxohydroxides have been identified repeatedly as superior electrocatalysts for the oxygen evolution reaction (OER), an exact description of the performance-relevant species has remained a challenge. In this context, we report the characterization of hydrothermally prepared iridium(III/IV) oxohydroxides that exhibit exceptional OER performances. Holes in the O 2p states of the iridium(III/IV) oxohydroxides result in reactive OI- species, which are identified by characteristic near-edge X-ray absorption fine structure (NEXAFS) features. A prototypical titration reaction with CO as a probe molecule shows that these OI- species are highly susceptible to nucleophilic attack at room temperature. Similarly to the preactivated oxygen involved in the biological OER in photosystem II, the electrophilic OI- species evidenced in the iridium(III/IV) oxohydroxides are suggested to be precursors to species involved in the O-O bond formation during the electrocatalytic OER. The CO titration also highlights a link between the OER performance and the surface/subsurface mobility of the OI- species. Thus, the superior electrocatalytic properties of the iridium (III/IV) oxohydroxides are explained by their ability to accommodate preactivated electrophilic OI- species that can migrate within the lattice.
Keywords: electrochemistry; energy storage; iridium; oxygen; water splitting.
© 2017 Wiley-VCH Verlag GmbH & Co. KGaA, Weinheim.
Figures







References
-
- None
-
- McCrory C. C. L., Jung S., Ferrer I. M., Chatman S. M., Peters J. C., Jaramillo T. F., J. Am. Chem. Soc. 2015, 137, 4347; - PubMed
-
- Katsounaros I., Cherevko S., Zeradjanin A. R., Mayrhofer K. J. J., Angew. Chem. Int. Ed. 2014, 53, 102; - PubMed
- Angew. Chem. 2014, 126, 104;
-
- Bernicke M., Ortel E., Reier T., Bergmann A., Ferreira de Araujo J., Strasser P., Kraehnert R., ChemSusChem 2015, 8, 1908. - PubMed
-
- Bockris J. O. M., Otagawa T., J. Electrochem. Soc. 1984, 131, 290.
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

