Activating α7 nicotinic acetylcholine receptor inhibits NLRP3 inflammasome through regulation of β-arrestin-1
- PMID: 28941191
- PMCID: PMC6492746
- DOI: 10.1111/cns.12758
Activating α7 nicotinic acetylcholine receptor inhibits NLRP3 inflammasome through regulation of β-arrestin-1
Abstract
Aims: To evaluate whether activating α7 nicotinic acetylcholine receptor (α7nAChR) could inhibit the NOD-like receptor family, pyrin domain containing 3 (NLRP3) inflammasome through regulation of β-arrestin-1 in monocyte/macrophage system, thus contributing to the control of neuroinflammation.
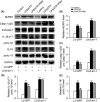
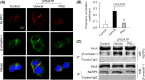
Methods: The protein levels of NLRP3, caspase-1 (Casp-1) p20 and proCasp-1, interleukin-1β (IL-1β) p17 and proIL-1β, IL-18 and proIL-18 were measured using Western blotting. The mRNA levels of Casp-1 and IL-1β were detected by real-time PCR (RT-PCR). The colocalization and interaction of NLRP3 protein and β-arrestin-1 were measured by immunofluorescence staining and immunoprecipitation.
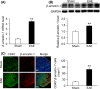
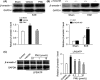
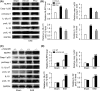
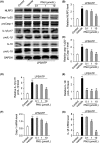
Results: The expression of β-arrestin-1 was significantly increased and colocalized with CD45-positive cells in spinal cord of experimental auto-immune encephalomyelitis (EAE) mice when compared with the sham mice, which was attenuated by pretreatment with PNU282987, a specific α7nAChR agonist. PNU282987 also significantly inhibited the activation of NLRP3 inflammasome and thus decreased the production of IL-1β and IL-18 both in lipopolysaccharide (LPS)/ATP-stimulated BV2 microglia in vitro and spinal cord from EAE mice in vivo, while inverse effects were observed in α7nAChR knockout mice. Furthermore, overexpression of β-arrestin-1 attenuated the inhibitory effect of PNU282987 on NLRP3 inflammasome activation in LPS/ATP-stimulated BV2 microglia. PNU282987 inhibited the interaction between β-arrestin-1 and NLRP3 protein in vitro.
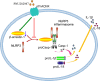
Conclusions: The present study demonstrates that activating α7nAChR can lead to NLRP3 inflammasome inhibition via regulation of β-arrestin-1 in monocyte/microglia system.
Keywords: NLRP3 inflammasome; neuroinflammation; α7nAChR; β-arrestin-1.
© 2017 John Wiley & Sons Ltd.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Similar articles
-
Activating cannabinoid receptor 2 alleviates pathogenesis of experimental autoimmune encephalomyelitis via activation of autophagy and inhibiting NLRP3 inflammasome.CNS Neurosci Ther. 2014 Dec;20(12):1021-8. doi: 10.1111/cns.12349. CNS Neurosci Ther. 2014. PMID: 25417929 Free PMC article.
-
Macrophage α7nAChR alleviates the inflammation of neonatal necrotizing enterocolitis through mTOR/NLRP3/IL-1β pathway.Int Immunopharmacol. 2024 Sep 30;139:112590. doi: 10.1016/j.intimp.2024.112590. Epub 2024 Jul 13. Int Immunopharmacol. 2024. PMID: 38996778
-
Benzyl isothiocyanate inhibits inflammasome activation in E. coli LPS-stimulated BV2 cells.Int J Mol Med. 2016 Sep;38(3):912-8. doi: 10.3892/ijmm.2016.2667. Epub 2016 Jul 6. Int J Mol Med. 2016. PMID: 27430883
-
Inflammasome activation in multiple sclerosis and experimental autoimmune encephalomyelitis (EAE).Brain Pathol. 2017 Mar;27(2):213-219. doi: 10.1111/bpa.12477. Brain Pathol. 2017. PMID: 27997058 Free PMC article. Review.
-
Mechanisms of NLRP3 Inflammasome Activation: Its Role in the Treatment of Alzheimer's Disease.Neurochem Res. 2020 Nov;45(11):2560-2572. doi: 10.1007/s11064-020-03121-z. Epub 2020 Sep 14. Neurochem Res. 2020. PMID: 32929691 Review.
Cited by
-
Cellular responses and functions of α7 nicotinic acetylcholine receptor activation in the brain: a narrative review.Ann Transl Med. 2021 Mar;9(6):509. doi: 10.21037/atm-21-273. Ann Transl Med. 2021. PMID: 33850906 Free PMC article. Review.
-
Rusty Microglia: Trainers of Innate Immunity in Alzheimer's Disease.Front Neurol. 2018 Dec 4;9:1062. doi: 10.3389/fneur.2018.01062. eCollection 2018. Front Neurol. 2018. PMID: 30564191 Free PMC article. Review.
-
Protective Effect of the Abelmoschus manihot Flower Extract on DSS-Induced Ulcerative Colitis in Mice.Evid Based Complement Alternat Med. 2021 Aug 9;2021:7422792. doi: 10.1155/2021/7422792. eCollection 2021. Evid Based Complement Alternat Med. 2021. PMID: 34408782 Free PMC article.
-
The Role of Neutrophil Extracellular Traps in Cancer.Front Oncol. 2021 Aug 12;11:714357. doi: 10.3389/fonc.2021.714357. eCollection 2021. Front Oncol. 2021. PMID: 34476216 Free PMC article. Review.
-
Emphasizing the Crosstalk Between Inflammatory and Neural Signaling in Post-traumatic Stress Disorder (PTSD).J Neuroimmune Pharmacol. 2023 Sep;18(3):248-266. doi: 10.1007/s11481-023-10064-z. Epub 2023 Apr 25. J Neuroimmune Pharmacol. 2023. PMID: 37097603 Free PMC article. Review.
References
-
- Liu C, Su D. Nicotinic acetylcholine receptor alpha7 subunit: a novel therapeutic target for cardiovascular diseases. Front Med. 2012;6:35‐40. - PubMed
-
- Liu C, Shen FM, Le YY, et al. Antishock effect of anisodamine involves a novel pathway for activating alpha7 nicotinic acetylcholine receptor. Crit Care Med. 2009;37:634‐641. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

