CDK5-dependent activation of dynein in the axon initial segment regulates polarized cargo transport in neurons
- PMID: 28941293
- PMCID: PMC5683723
- DOI: 10.1111/tra.12529
CDK5-dependent activation of dynein in the axon initial segment regulates polarized cargo transport in neurons
Abstract
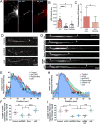
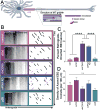
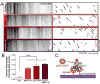
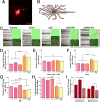
The unique polarization of neurons depends on selective sorting of axonal and somatodendritic cargos to their correct compartments. Axodendritic sorting and filtering occurs within the axon initial segment (AIS). However, the underlying molecular mechanisms responsible for this filter are not well understood. Here, we show that local activation of the neuronal-specific kinase cyclin-dependent kinase 5 (CDK5) is required to maintain AIS integrity, as depletion or inhibition of CDK5 induces disordered microtubule polarity and loss of AIS cytoskeletal structure. Furthermore, CDK5-dependent phosphorylation of the dynein regulator Ndel1 is required for proper re-routing of mislocalized somatodendritic cargo out of the AIS; inhibition of this pathway induces profound mis-sorting defects. While inhibition of the CDK5-Ndel1-Lis1-dynein pathway alters both axonal microtubule polarity and axodendritic sorting, we found that these defects occur on distinct timescales; brief inhibition of dynein disrupts axonal cargo sorting before loss of microtubule polarity becomes evident. Together, these studies identify CDK5 as a master upstream regulator of trafficking in vertebrate neurons, required for both AIS microtubule organization and polarized dynein-dependent sorting of axodendritic cargos, and support an ongoing and essential role for dynein at the AIS.
Keywords: CDK5; Lis1; Ndel1; axon initial segment; dynein; microtubule polarity; polarized transport.
© 2017 John Wiley & Sons A/S. Published by John Wiley & Sons Ltd.
Conflict of interest statement
The authors have no conflict of interest to declare.
Figures


References
-
- Nakada C, Ritchie K, Oba Y, et al. Accumulation of anchored proteins forms membrane diffusion barriers during neuronal polarization. Nat Cell Biol. 2003;5(7):626–633. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

