Semaglutide improves postprandial glucose and lipid metabolism, and delays first-hour gastric emptying in subjects with obesity
- PMID: 28941314
- PMCID: PMC5836914
- DOI: 10.1111/dom.13120
Semaglutide improves postprandial glucose and lipid metabolism, and delays first-hour gastric emptying in subjects with obesity
Abstract
Aim: To investigate the effects of semaglutide on fasting and postprandial glucose and lipid responses, and on gastric emptying.
Materials and methods: This was a randomized, double-blind, placebo-controlled, 2-period, crossover trial. Subjects with obesity (N = 30) received once-weekly subcutaneous semaglutide, dose-escalated to 1.0 mg, or placebo. After each 12-week treatment period, glucose and lipid metabolism were assessed before and after standardized meals. Gastric emptying (paracetamol absorption test) and peptide YY (PYY) response were also assessed.
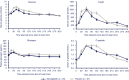
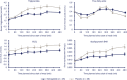
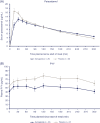
Results: Semaglutide treatment significantly lowered fasting concentrations of glucose and glucagon, and increased insulin vs placebo (estimated treatment ratio: 0.95 [95% confidence interval: 0.91, 0.98]; 0.86 [0.75, 0.98]; 1.45 [1.20, 1.75], respectively). Postprandial glucose metabolism significantly improved with semaglutide vs placebo (incremental area under the curve 0 to 5 hours [iAUC0-5h ]; estimated treatment difference: glucose -1.34 mmol h/L [-2.42, -0.27]; insulin -921 pmol h/L [-1461, -381]; C-peptide -1.42 nmol h/L [-2.33, -0.51]). Fasting and postprandial lipid metabolism improved with semaglutide vs placebo. First-hour gastric emptying after the meal was delayed with semaglutide vs placebo (AUC0-1h ; estimated treatment ratio: 0.73 [0.61, 0.87]); this may have contributed to the lower postprandial glucose increase in semaglutide-treated subjects. Overall gastric emptying (AUC0-5h ) was not statistically different between treatments. Fasting and postprandial PYY responses were significantly lower with semaglutide vs placebo (P = .0397 and P = .0097, respectively).
Conclusion: Semaglutide improved fasting and postprandial glucose and lipid metabolism. Overall gastric emptying was similar to that with placebo; however, the observed first-hour delay with semaglutide may contribute to a slower entry of glucose into the circulation.
Keywords: GLP-1 analogue; glucose metabolism; incretin therapy; insulin analogues; obesity therapy; phase I-II study.
© 2017 The Authors. Diabetes, Obesity and Metabolism published by John Wiley & Sons Ltd.
Conflict of interest statement
M. B. A., A. F., J. B. H. and T. K. are full‐time employees of, and hold shares in, Novo Nordisk A/S. A. B. has received research grants from Novo Nordisk. J. B. has received research, travel and accommodation grants within the submitted work from Novo Nordisk A/S, and advisory and speaker fees outside the submitted work from Novo Nordisk A/S.
Figures
Similar articles
-
Oral semaglutide improves postprandial glucose and lipid metabolism, and delays gastric emptying, in subjects with type 2 diabetes.Diabetes Obes Metab. 2021 Jul;23(7):1594-1603. doi: 10.1111/dom.14373. Epub 2021 Mar 29. Diabetes Obes Metab. 2021. PMID: 33710717 Free PMC article. Clinical Trial.
-
The once-daily human glucagon-like peptide-1 (GLP-1) analog liraglutide improves postprandial glucose levels in type 2 diabetes patients.Adv Ther. 2011 Mar;28(3):213-26. doi: 10.1007/s12325-010-0110-x. Epub 2011 Feb 17. Adv Ther. 2011. PMID: 21340616 Clinical Trial.
-
The effect of semaglutide 2.4 mg once weekly on energy intake, appetite, control of eating, and gastric emptying in adults with obesity.Diabetes Obes Metab. 2021 Mar;23(3):754-762. doi: 10.1111/dom.14280. Epub 2021 Jan 3. Diabetes Obes Metab. 2021. PMID: 33269530 Free PMC article. Clinical Trial.
-
Sgemaglutide in type 2 diabetes - is it the best glucagon-like peptide 1 receptor agonist (GLP-1R agonist)?Expert Opin Drug Metab Toxicol. 2018 Mar;14(3):371-377. doi: 10.1080/17425255.2018.1441286. Epub 2018 Feb 23. Expert Opin Drug Metab Toxicol. 2018. PMID: 29439603 Review.
-
[New outcome studies with injectable semaglutide in different at risk populations].Rev Med Liege. 2024 Oct;79(10):676-682. Rev Med Liege. 2024. PMID: 39397557 Review. French.
Cited by
-
Efficacy and safety of semaglutide on weight loss in obese or overweight patients without diabetes: A systematic review and meta-analysis of randomized controlled trials.Front Pharmacol. 2022 Sep 14;13:935823. doi: 10.3389/fphar.2022.935823. eCollection 2022. Front Pharmacol. 2022. PMID: 36188627 Free PMC article.
-
Carbohydrate-insulin model: does the conventional view of obesity reverse cause and effect?Philos Trans R Soc Lond B Biol Sci. 2023 Oct 23;378(1888):20220211. doi: 10.1098/rstb.2022.0211. Epub 2023 Sep 4. Philos Trans R Soc Lond B Biol Sci. 2023. PMID: 37661740 Free PMC article. Review.
-
The safety and efficacy of once-weekly glucagon-like peptide-1 receptor agonist semaglutide in patients with type 2 diabetes mellitus: a systemic review and meta-analysis.Endocrine. 2018 Dec;62(3):535-545. doi: 10.1007/s12020-018-1708-z. Epub 2018 Aug 12. Endocrine. 2018. PMID: 30101378
-
A Pharmacological and Clinical Overview of Oral Semaglutide for the Treatment of Type 2 Diabetes.Drugs. 2021 Jun;81(9):1003-1030. doi: 10.1007/s40265-021-01499-w. Epub 2021 May 8. Drugs. 2021. PMID: 33964002 Free PMC article. Review.
-
Gastrointestinal Motility Effects of GLP-1 Receptor Agonists.Curr Gastroenterol Rep. 2025 Jul 7;27(1):49. doi: 10.1007/s11894-025-00995-3. Curr Gastroenterol Rep. 2025. PMID: 40622491 Review.
References
-
- International Diabetes Federation . IDF Diabetes Atlas. 7th ed. IDF Web site. www.diabetesatlas.org/component/attachments/?task=download&id=116. IDF, 2015. Accessed August 16, 2017.
-
- American Diabetes Association . Classification and diagnosis of diabetes. Diabetes Care. 2016;39:S13–S22. - PubMed
-
- Raccah D, Chou E, Colagiuri S, et al. A global study of the unmet need for glycemic control and predictor factors among patients with type 2 diabetes mellitus who have achieved optimal fasting plasma glucose control on basal insulin. Diabetes Metab Res Rev. 2017;33: https://doi.org/10.1002/dmrr.2858. [Epub 13 Oct 2016]. - DOI - PMC - PubMed
-
- American Diabetes Association . Cardiovascular disease and risk management. Diabetes Care. 2017;40:S75–S87. - PubMed
-
- Stone NJ, Robinson JG, Lichtenstein AH, et al; American College of Cardiology/American Heart Association Task Force on Practice Guidelines. 2013 ACC/AHA guideline on the treatment of blood cholesterol to reduce atherosclerotic cardiovascular risk in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation. 2014;129:S1–S45. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

