Neuro-immune interactions in inflammation and host defense: Implications for transplantation
- PMID: 28941325
- PMCID: PMC5820210
- DOI: 10.1111/ajt.14515
Neuro-immune interactions in inflammation and host defense: Implications for transplantation
Abstract
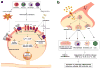
Sensory and autonomic neurons of the peripheral nervous system (PNS) play a critical role in regulating the immune system during tissue inflammation and host defense. Recent studies have identified the molecular mechanisms underlying the bidirectional communication between the nervous system and the immune system. Here, we highlight the studies that demonstrate the importance of the neuro-immune interactions in health and disease. Nociceptor sensory neurons detect immune mediators to produce pain, and release neuropeptides that act on the immune system to regulate inflammation. In parallel, neural reflex circuits including the vagus nerve-based inflammatory reflex are physiological regulators of inflammatory responses and cytokine production. In transplantation, neuro-immune communication could significantly impact the processes of host-pathogen defense, organ rejection, and wound healing. Emerging approaches to target the PNS such as bioelectronics could be useful in improving the outcome of transplantation. Therefore, understanding how the nervous system shapes the immune response could have important therapeutic ramifications for transplantation medicine.
Keywords: basic (laboratory) research/science; immune regulation; immunosuppression/immune modulation; innate immunity; neurology.
© 2017 The American Society of Transplantation and the American Society of Transplant Surgeons.
Conflict of interest statement
The authors of this manuscript have no conflicts of interest to disclose as described by the
Figures

Similar articles
-
The Microbiome and Immune Regulation After Transplantation.Transplantation. 2017 Jan;101(1):56-62. doi: 10.1097/TP.0000000000001444. Transplantation. 2017. PMID: 27517729 Free PMC article. Review.
-
Insights on the impact of diet-mediated microbiota alterations on immunity and diseases.Am J Transplant. 2018 Mar;18(3):550-555. doi: 10.1111/ajt.14477. Epub 2017 Sep 23. Am J Transplant. 2018. PMID: 28858426 Review.
-
Neural Immune Communication in the Control of Host-Bacterial Pathogen Interactions in the Gastrointestinal Tract.Infect Immun. 2020 Aug 19;88(9):e00928-19. doi: 10.1128/IAI.00928-19. Print 2020 Aug 19. Infect Immun. 2020. PMID: 32341116 Free PMC article. Review.
-
Neuro-immune interactions in allergic diseases: novel targets for therapeutics.Int Immunol. 2017 Jun 1;29(6):247-261. doi: 10.1093/intimm/dxx040. Int Immunol. 2017. PMID: 28814067 Free PMC article.
-
Neuro-immune Interactions in the Tissues.Immunity. 2020 Mar 17;52(3):464-474. doi: 10.1016/j.immuni.2020.02.017. Immunity. 2020. PMID: 32187517 Free PMC article. Review.
Cited by
-
Interleukin-10 signaling in somatosensory neurons controls CCL2 release and inflammatory response.Brain Behav Immun. 2024 Feb;116:193-202. doi: 10.1016/j.bbi.2023.12.013. Epub 2023 Dec 9. Brain Behav Immun. 2024. PMID: 38081433 Free PMC article. Review.
-
Interleukin-17 as a spatiotemporal bridge from acute to chronic inflammation: Novel insights from computational modeling.WIREs Mech Dis. 2023 May-Jun;15(3):e1599. doi: 10.1002/wsbm.1599. Epub 2023 Jan 29. WIREs Mech Dis. 2023. PMID: 36710253 Free PMC article.
-
Jmjd3 Mediates Neuropathic Pain by Inducing Macrophage Infiltration and Activation in Lumbar Spinal Stenosis Animal Model.Int J Mol Sci. 2021 Dec 14;22(24):13426. doi: 10.3390/ijms222413426. Int J Mol Sci. 2021. PMID: 34948220 Free PMC article.
-
Role of Peripheral Immune Cells for Development and Recovery of Chronic Pain.Front Immunol. 2021 Feb 22;12:641588. doi: 10.3389/fimmu.2021.641588. eCollection 2021. Front Immunol. 2021. PMID: 33692810 Free PMC article. Review.
-
HMGB1 released from nociceptors mediates inflammation.Proc Natl Acad Sci U S A. 2021 Aug 17;118(33):e2102034118. doi: 10.1073/pnas.2102034118. Proc Natl Acad Sci U S A. 2021. PMID: 34385304 Free PMC article.
References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

