Gestational respiratory infections interacting with offspring HLA and CTLA-4 modifies incident β-cell autoantibodies
- PMID: 28941965
- PMCID: PMC5747989
- DOI: 10.1016/j.jaut.2017.09.005
Gestational respiratory infections interacting with offspring HLA and CTLA-4 modifies incident β-cell autoantibodies
Abstract
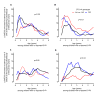
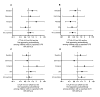
β-cell autoantibodies against insulin (IAA), GAD65 (GADA) and IA-2 (IA-2A) precede onset of childhood type 1 diabetes (T1D). Incidence of the first appearing β-cell autoantibodies peaks at a young age and is patterned by T1D-associated genes, suggesting an early environmental influence. Here, we tested if gestational infections and interactions with child's human leukocyte antigen (HLA) and non-HLA genes affected the appearance of the first β-cell autoantibody. Singletons of mothers without diabetes (n = 7472) with T1D-associated HLA-DR-DQ genotypes were prospectively followed quarterly through the first 4 years of life, then semiannually until age 6 years, using standardized autoantibody analyses. Maternal infections during pregnancy were assessed via questionnaire 3-4.5 months post-delivery. Polymorphisms in twelve non-HLA genes associated with the first appearing β-cell autoantibodies were included in a Cox regression analysis. IAA predominated as the first appearing β-cell autoantibody in younger children (n = 226, median age at seroconversion 1.8 years) and GADA (n = 212; 3.2 years) in children aged ≥2 years. Gestational infections were not associated with the first appearing β-cell autoantibodies overall. However, gestational respiratory infections (G-RI) showed a consistent protective influence on IAA (HR 0.64, 95% CI 0.45-0.91) among CTLA4-(AG, GG) children (G-RI*CTLA4 interaction, p = 0.002). The predominant associations of HLA-DR-DQ 4-8/8-4 with IAA and HLA-DR-DQ 3-2/3-2 with GADA were not observed if a G-RI was reported (G-RI*HLA-DR-DQ interaction, p = 0.03). The role of G-RI may depend on offspring HLA and CTLA-4 alleles and supports a bidirectional trigger for IAA or GADA as a first appearing β-cell autoantibody in early life.
Keywords: Autoimmune diabetes; Autoimmunity; Glutamic acid decarboxylase; HLA; IA-2; Insulin; Type 1 diabetes; β-cell autoantibodies.
Copyright © 2017 Elsevier Ltd. All rights reserved.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- Hagopian WA, Erlich H, Lernmark A, Rewers M, Ziegler AG, Simell O, Akolkar B, Vogt R, Jr, Blair A, Ilonen J, et al. The Environmental Determinants of Diabetes in the Young (TEDDY): genetic criteria and international diabetes risk screening of 421 000 infants. Pediatric Diabetes. 2011;12(8):733–43. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- U01 DK063821/DK/NIDDK NIH HHS/United States
- UC4 DK063863/DK/NIDDK NIH HHS/United States
- HHSN267200700014C/DK/NIDDK NIH HHS/United States
- U01 DK063861/DK/NIDDK NIH HHS/United States
- UL1 TR001427/TR/NCATS NIH HHS/United States
- U01 DK063790/DK/NIDDK NIH HHS/United States
- UL1 TR001082/TR/NCATS NIH HHS/United States
- P30 DK017047/DK/NIDDK NIH HHS/United States
- UL1 TR000064/TR/NCATS NIH HHS/United States
- U01 DK063836/DK/NIDDK NIH HHS/United States
- U01 DK063829/DK/NIDDK NIH HHS/United States
- U01 DK063865/DK/NIDDK NIH HHS/United States
- UC4 DK095300/DK/NIDDK NIH HHS/United States
- UC4 DK063861/DK/NIDDK NIH HHS/United States
- UC4 DK063829/DK/NIDDK NIH HHS/United States
- UC4 DK063821/DK/NIDDK NIH HHS/United States
- UC4 DK117483/DK/NIDDK NIH HHS/United States
- UC4 DK063836/DK/NIDDK NIH HHS/United States
- UC4 DK112243/DK/NIDDK NIH HHS/United States
- UC4 DK063865/DK/NIDDK NIH HHS/United States
- U01 DK063863/DK/NIDDK NIH HHS/United States
- UC4 DK106955/DK/NIDDK NIH HHS/United States
- UC4 DK100238/DK/NIDDK NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

