Pallidal low β-low γ phase-amplitude coupling inversely correlates with Parkinson disease symptoms
- PMID: 28942154
- PMCID: PMC5675765
- DOI: 10.1016/j.clinph.2017.08.001
Pallidal low β-low γ phase-amplitude coupling inversely correlates with Parkinson disease symptoms
Abstract
Objective: Recent discoveries suggest that it is most likely the coupling of β oscillations (13-30Hz) and not merely their power that relates to Parkinson disease (PD) pathophysiology.
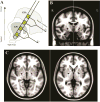
Methods: We analyzed power and phase amplitude coupling (PAC) in local field potentials (LFP) recorded from Pallidum after placement of deep brain stimulation (DBS) leads in nineteen PD patients and three patients with dystonia.
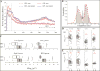
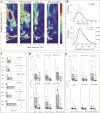
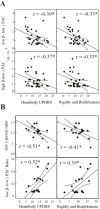
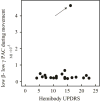
Results: Within GPi, we identified PAC between phase of β and amplitude of high frequency oscillations (200-300Hz) and distinct β-low γ (40-80Hz) PAC both modulated by contralateral movement. Resting β-low γ PAC, also present in dystonia patients, inversely correlated with severity of rigidity and bradykinesia (R=-0.44, P=0.028). These findings were specific to the low β band, suggesting a differential role for the two β sub-bands.
Conclusions: PAC is present across distinct frequency bands within the GPi. Given the presence of low β-low γ PAC in dystonia and the inverse correlation with symptom severity, we propose that this PAC may be a normal pallidal signal.
Significance: This study provides new evidence on the pathophysiological contribution of local pallidal coupling and suggests similar and distinct patterns of coupling within GPi and STN in PD.
Keywords: Basal ganglia; Deep brain stimulation; Parkinson disease; Phase-amplitude coupling; β oscillations.
Copyright © 2017 International Federation of Clinical Neurophysiology. Published by Elsevier B.V. All rights reserved.
Conflict of interest statement
None of the authors have potential conflicts of interest to be disclosed.
Figures
References
-
- Andersson JLR, Jenkinson M, Smith S. Non-linear registration aka Spatial normalisation. FMRIB Technial Report TR07JA2. 2007;22 In Pract.
-
- Benjamini Y, Drai D, Elmer G, Kafkafi N, Golani I. Controlling the false discovery rate in behavior genetics research. Behav Brain Res. 2001;125:279–84. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

