Re-examining the arterial cord blood gas pH screening criteria in neonatal encephalopathy
- PMID: 28942435
- PMCID: PMC6192544
- DOI: 10.1136/archdischild-2017-313078
Re-examining the arterial cord blood gas pH screening criteria in neonatal encephalopathy
Abstract
Objective: Screening criteria for neonatal encephalopathy remain a complex combination of subjective and objective criteria. We examine the utility of universal cord blood gas testing and mandatory encephalopathy evaluation for infants with pH ≤7.10 on umbilical cord arterial blood gas (cABG) as a single screening measure for timely identification of moderate/severe encephalopathy.
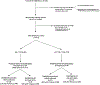
Design, setting, patients: Infants born at a single centre between 2008 and 2015, who were ≥36 weeks, had no congenital anomalies and had a cABG pH ≤7.10 were identified for a retrospective cohort study. Maternal/perinatal and patient factors were collected.
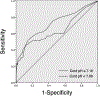
Results: 27 028 infants were born during the study period; 412 met all inclusion criteria. Of those, 35/85 infants with pH <7.00 and 34/327 infants with pH between 7.00 and 7.10 had moderate/severe encephalopathy. Encephalopathy was identified on the basis of pH and examination alone (no other perinatal criteria present) in 5/35 and 13/34 infants in the two pH groups, respectively.A cABG pH threshold of ≤7.10 was associated with a sensitivity of 74.2% and a specificity of 98.7% for detection of moderate/severe encephalopathy. Based on these data, 25 infants with cABG pH between 7.00 and 7.10 will need to be screened to identify one neonate with moderate/severe encephalopathy, who might have otherwise been missed using conventional screening, a 15% increase in appropriate selection and treatment over current methods.
Conclusion: Universal cord blood gas screening with a pH threshold ≤7.10 and mandatory encephalopathy examination results in greater detection of infants with moderate/severe encephalopathy and timely initiation of therapeutic hypothermia.
Keywords: neonatology; neurology.
© Article author(s) (or their employer(s) unless otherwise stated in the text of the article) 2018. All rights reserved. No commercial use is permitted unless otherwise expressly granted.
Conflict of interest statement
Competing interests: None declared.
Figures
References
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous