Age-Dependent Alterations in Meiotic Recombination Cause Chromosome Segregation Errors in Spermatocytes
- PMID: 28942922
- PMCID: PMC5679441
- DOI: 10.1016/j.cell.2017.08.042
Age-Dependent Alterations in Meiotic Recombination Cause Chromosome Segregation Errors in Spermatocytes
Abstract
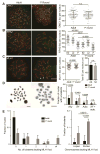
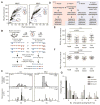
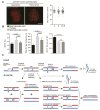
Faithful chromosome segregation in meiosis requires crossover (CO) recombination, which is regulated to ensure at least one CO per homolog pair. We investigate the failure to ensure COs in juvenile male mice. By monitoring recombination genome-wide using cytological assays and at hotspots using molecular assays, we show that juvenile mouse spermatocytes have fewer COs relative to adults. Analysis of recombination in the absence of MLH3 provides evidence for greater utilization in juveniles of pathways involving structure-selective nucleases and alternative complexes, which can act upon precursors to generate noncrossovers (NCOs) at the expense of COs. We propose that some designated CO sites fail to mature efficiently in juveniles owing to inappropriate activity of these alternative repair pathways, leading to chromosome mis-segregation. We also find lower MutLγ focus density in juvenile human spermatocytes, suggesting that weaker CO maturation efficiency may explain why younger men have a higher risk of fathering children with Down syndrome.
Keywords: DNA repair; aneuploidy; chromosome segregation; crossing over; gene conversion; germ cells; homologous recombination; meiosis; noncrossovers; spermatocytes.
Copyright © 2017 Elsevier Inc. All rights reserved.
Figures


Similar articles
-
A mutation in the endonuclease domain of mouse MLH3 reveals novel roles for MutLγ during crossover formation in meiotic prophase I.PLoS Genet. 2019 Jun 6;15(6):e1008177. doi: 10.1371/journal.pgen.1008177. eCollection 2019 Jun. PLoS Genet. 2019. PMID: 31170160 Free PMC article.
-
SHOC1 is a ERCC4-(HhH)2-like protein, integral to the formation of crossover recombination intermediates during mammalian meiosis.PLoS Genet. 2018 May 9;14(5):e1007381. doi: 10.1371/journal.pgen.1007381. eCollection 2018 May. PLoS Genet. 2018. PMID: 29742103 Free PMC article.
-
Mammalian CNTD1 is critical for meiotic crossover maturation and deselection of excess precrossover sites.J Cell Biol. 2014 Jun 9;205(5):633-41. doi: 10.1083/jcb.201401122. Epub 2014 Jun 2. J Cell Biol. 2014. PMID: 24891606 Free PMC article.
-
Female Meiosis: Synapsis, Recombination, and Segregation in Drosophila melanogaster.Genetics. 2018 Mar;208(3):875-908. doi: 10.1534/genetics.117.300081. Genetics. 2018. PMID: 29487146 Free PMC article. Review.
-
The spatial regulation of meiotic recombination hotspots: are all DSB hotspots crossover hotspots?Exp Cell Res. 2012 Jul 15;318(12):1347-52. doi: 10.1016/j.yexcr.2012.03.025. Epub 2012 Mar 31. Exp Cell Res. 2012. PMID: 22487095 Review.
Cited by
-
Maternal CENP-C restores centromere symmetry in mammalian zygotes to ensure proper chromosome segregation.bioRxiv [Preprint]. 2025 Jul 28:2025.07.23.666394. doi: 10.1101/2025.07.23.666394. bioRxiv. 2025. PMID: 40766472 Free PMC article. Preprint.
-
Genetic dissection of crossover mutants defines discrete intermediates in mouse meiosis.Mol Cell. 2023 Aug 17;83(16):2941-2958.e7. doi: 10.1016/j.molcel.2023.07.022. Mol Cell. 2023. PMID: 37595556 Free PMC article.
-
shani mutation in mouse affects splicing of Spata22 and leads to impaired meiotic recombination.Chromosoma. 2020 Jun;129(2):161-179. doi: 10.1007/s00412-020-00735-8. Epub 2020 May 10. Chromosoma. 2020. PMID: 32388826 Free PMC article.
-
A rigorous measure of genome-wide genetic shuffling that takes into account crossover positions and Mendel's second law.Proc Natl Acad Sci U S A. 2019 Jan 29;116(5):1659-1668. doi: 10.1073/pnas.1817482116. Epub 2019 Jan 11. Proc Natl Acad Sci U S A. 2019. PMID: 30635424 Free PMC article.
-
Cell-type-specific genomics reveals histone modification dynamics in mammalian meiosis.Nat Commun. 2019 Aug 23;10(1):3821. doi: 10.1038/s41467-019-11820-7. Nat Commun. 2019. PMID: 31444359 Free PMC article.
References
-
- Borner GV, Kleckner N, Hunter N. Crossover/noncrossover differentiation, synaptonemal complex formation, and regulatory surveillance at the leptotene/zygotene transition of meiosis. Cell. 2004;117:29–45. - PubMed
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

