Deconstruction of Corticospinal Circuits for Goal-Directed Motor Skills
- PMID: 28942925
- PMCID: PMC5679421
- DOI: 10.1016/j.cell.2017.08.014
Deconstruction of Corticospinal Circuits for Goal-Directed Motor Skills
Abstract
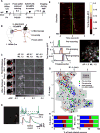
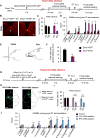
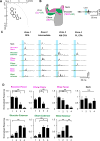
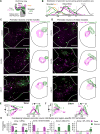
Corticospinal neurons (CSNs) represent the direct cortical outputs to the spinal cord and play important roles in motor control across different species. However, their organizational principle remains unclear. By using a retrograde labeling system, we defined the requirement of CSNs in the execution of a skilled forelimb food-pellet retrieval task in mice. In vivo imaging of CSN activity during performance revealed the sequential activation of topographically ordered functional ensembles with moderate local mixing. Region-specific manipulations indicate that CSNs from caudal or rostral forelimb area control reaching or grasping, respectively, and both are required in the transitional pronation step. These region-specific CSNs terminate in different spinal levels and locations, therefore preferentially connecting with the premotor neurons of muscles engaged in different steps of the task. Together, our findings suggest that spatially defined groups of CSNs encode different movement modules, providing a logic for parallel-ordered corticospinal circuits to orchestrate multistep motor skills.
Keywords: corticospinal neurons; food-pellet retrieval task; goal-directed motor skills; in vivo imaging; motor cortex; muscle control; premotor neurons.
Copyright © 2017 Elsevier Inc. All rights reserved.
Figures



References
-
- Ayling OG, Harrison TC, Boyd JD, Goroshkov A, Murphy TH. Automated light-based mapping of motor cortex by photoactivation of channelrhodopsin-2 transgenic mice. Nature methods. 2009;6:219–224. - PubMed
-
- Bareyre FM, Kerschensteiner M, Misgeld T, Sanes JR. Transgenic labeling of the corticospinal tract for monitoring axonal responses to spinal cord injury. Nat Med. 2005;11:1355–1360. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

