NG2 antigen is involved in leukemia invasiveness and central nervous system infiltration in MLL-rearranged infant B-ALL
- PMID: 28943635
- PMCID: PMC5843903
- DOI: 10.1038/leu.2017.294
NG2 antigen is involved in leukemia invasiveness and central nervous system infiltration in MLL-rearranged infant B-ALL
Erratum in
-
Correction: NG2 antigen is involved in leukemia invasiveness and central nervous system infiltration in MLL-rearranged infant B-ALL.Leukemia. 2018 Oct;32(10):2306. doi: 10.1038/s41375-018-0236-4. Leukemia. 2018. PMID: 30218009 Free PMC article.
Abstract
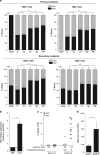
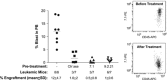
Mixed-lineage leukemia (MLL)-rearranged (MLLr) infant B-cell acute lymphoblastic leukemia (iMLLr-B-ALL) has a dismal prognosis and is associated with a pro-B/mixed phenotype, therapy refractoriness and frequent central nervous system (CNS) disease/relapse. Neuron-glial antigen 2 (NG2) is specifically expressed in MLLr leukemias and is used in leukemia immunophenotyping because of its predictive value for MLLr acute leukemias. NG2 is involved in melanoma metastasis and brain development; however, its role in MLL-mediated leukemogenesis remains elusive. Here we evaluated whether NG2 distinguishes leukemia-initiating/propagating cells (L-ICs) and/or CNS-infiltrating cells (CNS-ICs) in iMLLr-B-ALL. Clinical data from the Interfant cohort of iMLLr-B-ALL demonstrated that high NG2 expression associates with lower event-free survival, higher number of circulating blasts and more frequent CNS disease/relapse. Serial xenotransplantation of primary MLL-AF4+ leukemias indicated that NG2 is a malleable marker that does not enrich for L-IC or CNS-IC in iMLLr-B-All. However, NG2 expression was highly upregulated in blasts infiltrating extramedullar hematopoietic sites and CNS, and specific blockage of NG2 resulted in almost complete loss of engraftment. Indeed, gene expression profiling of primary blasts and primografts revealed a migratory signature of NG2+ blasts. This study provides new insights on the biology of NG2 in iMLLr-B-ALL and suggests NG2 as a potential therapeutic target to reduce the risk of CNS disease/relapse and to provide safer CNS-directed therapies for iMLLr-B-ALL.
Conflict of interest statement
The authors declare no conflict of interest.
Figures





Similar articles
-
NG2 antigen is a therapeutic target for MLL-rearranged B-cell acute lymphoblastic leukemia.Leukemia. 2019 Jul;33(7):1557-1569. doi: 10.1038/s41375-018-0353-0. Epub 2019 Jan 11. Leukemia. 2019. PMID: 30635633 Free PMC article.
-
NG2 is a target gene of MLL-AF4 and underlies glucocorticoid resistance in MLLr B-ALL by regulating NR3C1 expression.Blood. 2024 Nov 7;144(19):2002-2017. doi: 10.1182/blood.2023022050. Blood. 2024. PMID: 39093982
-
Insights into the cellular origin and etiology of the infant pro-B acute lymphoblastic leukemia with MLL-AF4 rearrangement.Leukemia. 2011 Mar;25(3):400-10. doi: 10.1038/leu.2010.284. Epub 2010 Dec 7. Leukemia. 2011. PMID: 21135858 Review.
-
The AF4-MLL fusion transiently augments multilineage hematopoietic engraftment but is not sufficient to initiate leukemia in cord blood CD34+ cells.Oncotarget. 2017 Jul 26;8(47):81936-81941. doi: 10.18632/oncotarget.19567. eCollection 2017 Oct 10. Oncotarget. 2017. PMID: 29137234 Free PMC article.
-
B-cell acute lymphoblastic leukemia with mature phenotype and MLL rearrangement: report of five new cases and review of the literature.Leuk Lymphoma. 2016 Oct;57(10):2289-97. doi: 10.3109/10428194.2016.1141407. Epub 2016 Feb 8. Leuk Lymphoma. 2016. PMID: 26857438 Review.
Cited by
-
A very rare case of FLT3-D835 positive blastic plasmacytoid dendritic cell neoplasm.Arch Clin Cases. 2021 Oct 27;7(4):57-62. doi: 10.22551/2020.29.0704.10174. eCollection 2020. Arch Clin Cases. 2021. PMID: 34754929 Free PMC article.
-
IMiDs mobilize acute myeloid leukemia blasts to peripheral blood through downregulation of CXCR4 but fail to potentiate AraC/Idarubicin activity in preclinical models of non del5q/5q- AML.Oncoimmunology. 2018 Jul 26;7(9):e1477460. doi: 10.1080/2162402X.2018.1477460. eCollection 2018. Oncoimmunology. 2018. PMID: 30228947 Free PMC article.
-
NG2 Molecule Expression in Acute Lymphoblastic Leukemia B Cells: A Flow-Cytometric Marker for the Rapid Identification of KMT2A Gene Rearrangements.Mediterr J Hematol Infect Dis. 2024 Mar 1;16(1):e2024018. doi: 10.4084/MJHID.2024.018. eCollection 2024. Mediterr J Hematol Infect Dis. 2024. PMID: 38468826 Free PMC article.
-
Insights into KMT2A rearrangements in acute myeloid leukemia: from molecular characteristics to targeted therapies.Biomark Res. 2025 May 13;13(1):73. doi: 10.1186/s40364-025-00786-y. Biomark Res. 2025. PMID: 40361241 Free PMC article. Review.
-
Integrative methylome-transcriptome analysis unravels cancer cell vulnerabilities in infant MLL-rearranged B cell acute lymphoblastic leukemia.J Clin Invest. 2021 Jul 1;131(13):e138833. doi: 10.1172/JCI138833. J Clin Invest. 2021. PMID: 33983906 Free PMC article.
References
-
- Thomas M, Gessner A, Vornlocher HP, Hadwiger P, Greil J, Heidenreich O. Targeting MLL-AF4 with short interfering RNAs inhibits clonogenicity and engraftment of t(4;11)-positive human leukemic cells. Blood 2005; 106: 3559–3566. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

