Microplate magnetic chemiluminescence immunoassay for detecting urinary survivin in bladder cancer
- PMID: 28943911
- PMCID: PMC5605963
- DOI: 10.3892/ol.2017.6675
Microplate magnetic chemiluminescence immunoassay for detecting urinary survivin in bladder cancer
Abstract
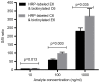
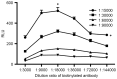
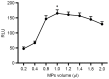
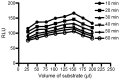
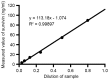
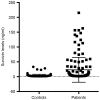
Survivin is a tumor marker for bladder cancer; however the role of urinary survivin levels has not been fully elucidated due to the limitations of current detection methods. Based on two survivin-specific monoclonal antibodies (McAbs) already confirmed through enzyme linked immunosorbent assays, the present study aimed to establish a microplate magnetic chemiluminescence immunoassay (CLIA) for the detection of urinary survivin levels and evaluate its application for the diagnosis of patients with bladder cancer. Horseradish peroxidase and biotin conjugates were used to label two different anti-survivin McAbs, respectively. The labeled antibodies combined with survivin to form a sandwiched immune complex. The streptavidin magnetic particles (MPs) served as the solid phase and the separator. The relevant parameters involved in the immunoassay, including the immunoassay reagents used and the physicochemical parameters were optimized. Then, urine samples from 130 patients with bladder cancer and 113 healthy controls were detected, and analyzed using the established method. The method was linear to 1,000 ng/ml survivin with a detection limit of 0.83 ng/ml. The intra- and inter-assay coefficients of variation were <8, and <11%, respectively. The concentration of diluted survivin and the dilution ratios gave a linear correlation of 0.9989. The results demonstrated that the urinary survivin levels in patients with bladder cancer were significantly higher (P<0.001) compared with that in healthy controls. At a survivin concentration of 2.0884 ng/ml, the sensitivity and specificity were 86.9 and 61.9%, respectively. Furthermore, the urinary survivin levels were positively correlated with metastatic stage, histological stage and recurrence (P<0.01). In conclusion, the present study preliminarily proposed a microplate magnetic CLIA for survivin detection and further evaluated the value of urinary survivin as a diagnostic marker for bladder cancer.
Keywords: bladder cancer; microplate magnetic chemiluminescence immuno-assay; survivin; tumor markers.
Figures




Similar articles
-
Detection of urinary survivin using a magnetic particles-based chemiluminescence immunoassay for the preliminary diagnosis of bladder cancer and renal cell carcinoma combined with LAPTM4B.Oncol Lett. 2018 May;15(5):7923-7933. doi: 10.3892/ol.2018.8317. Epub 2018 Mar 22. Oncol Lett. 2018. PMID: 29725479 Free PMC article.
-
A rapid and sensitive chemiluminescence immunoassay based on magnetic particles for squamous cell carcinoma antigen in human serum.Clin Chim Acta. 2011 Aug 17;412(17-18):1572-7. doi: 10.1016/j.cca.2011.05.005. Epub 2011 May 12. Clin Chim Acta. 2011. PMID: 21600892
-
Sandwich ELISA for detecting urinary Survivin in bladder cancer.Chin J Cancer Res. 2013 Aug;25(4):375-81. doi: 10.3978/j.issn.1000-9604.2013.08.11. Chin J Cancer Res. 2013. PMID: 23997523 Free PMC article.
-
Diagnostic value of urinary survivin as a biomarker for bladder cancer: a systematic review and meta-analysis of published studies.World J Urol. 2018 Sep;36(9):1373-1381. doi: 10.1007/s00345-018-2285-8. Epub 2018 Apr 2. World J Urol. 2018. PMID: 29610963
-
Bladder cancer detection with urinary survivin, an inhibitor of apoptosis.Front Biosci. 2002 Feb 1;7:e36-41. doi: 10.2741/sharp. Front Biosci. 2002. PMID: 11815300 Review.
Cited by
-
A Review of Biosensors for Detecting Tumor Markers in Breast Cancer.Life (Basel). 2022 Feb 25;12(3):342. doi: 10.3390/life12030342. Life (Basel). 2022. PMID: 35330093 Free PMC article. Review.
-
Diagnostic accuracy of urinary survivin mRNA expression detected by RT-PCR compared with urine cytology in the detection of bladder cancer: A meta-analysis of diagnostic test accuracy in head-to-head studies.Oncol Lett. 2020 Feb;19(2):1165-1174. doi: 10.3892/ol.2019.11227. Epub 2019 Dec 18. Oncol Lett. 2020. PMID: 31966046 Free PMC article.
-
Urinary expression of genes involved in DNA methylation and histone modification for diagnosis of bladder cancer in patients with asymptomatic microscopic haematuria.Oncol Lett. 2019 Jul;18(1):57-62. doi: 10.3892/ol.2019.10330. Epub 2019 May 7. Oncol Lett. 2019. PMID: 31289472 Free PMC article.
-
Magnetic Particle-Based Automated Chemiluminescence Immunoassay for the Determination of Hydrocortisone Residues in Milk.Foods. 2025 Jun 16;14(12):2105. doi: 10.3390/foods14122105. Foods. 2025. PMID: 40565714 Free PMC article.
-
Detection of urinary survivin using a magnetic particles-based chemiluminescence immunoassay for the preliminary diagnosis of bladder cancer and renal cell carcinoma combined with LAPTM4B.Oncol Lett. 2018 May;15(5):7923-7933. doi: 10.3892/ol.2018.8317. Epub 2018 Mar 22. Oncol Lett. 2018. PMID: 29725479 Free PMC article.
References
-
- Lokeshwar VB, Habuchi T, Grossman HB, Murphy WM, Hautmann SH, Hemstreet GP, III, Bono AV, Getzenberg RH, Goebell P, Schmitz-Dräger BJ, et al. Bladder tumor markers beyond cytology: International consensus panel on bladder tumor markers. Urology. 2005;66:S35–S63. doi: 10.1016/j.urology.2005.08.064. (6 Suppl 1) - DOI - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
